Chapter 10 Motor System
10.7 Neurodegenerative Diseases: Motor
Neurodegenerative diseases cause neurons to lose either their function or structure over time, potentially leading to cell death in either the central or peripheral nervous system. These diseases can be devastating because there are no known ways to reverse the neurodegeneration. There are multiple diseases that are classified as neurodegenerative diseases and affect millions of people worldwide. We will focus on the two most prevalent neurodegenerative diseases: The motor disease Parkinson’s disease that affects the basal ganglia, and Alzheimer’s disease.
Parkinson’s Disease
Symptoms and Risk Factors
Parkinson’s disease is a neurodegenerative movement disorder that causes bradykinesia (slowness of movement), akinesia (difficulty initiating movement), a resting tremor, muscle rigidity, and changes to posture and locomotion. Although most symptoms are motor, there are mild cognitive and psychiatric changes, such as apathy, anhedonia, mood disturbances, or depression.
Advanced age is the primary risk factor, as an estimated 2% of people over the age of 60 develop Parkinson’s disease. It is also shows a sex difference with men being diagnosed more than women. Other environmental factors contribute to risk, such as repeated traumatic brain injury (suspected in Muhammad Ali) or occupational exposure to heavy metals, insecticides, or other neurotoxins. A small percentage of cases are early onset (21 – 50 years old; Michael J. Fox was diagnosed at 30), and have a strong genetic component. The disease causes significant decreases in life expectancy and quality of life.
Basal Ganglia Dysfunction
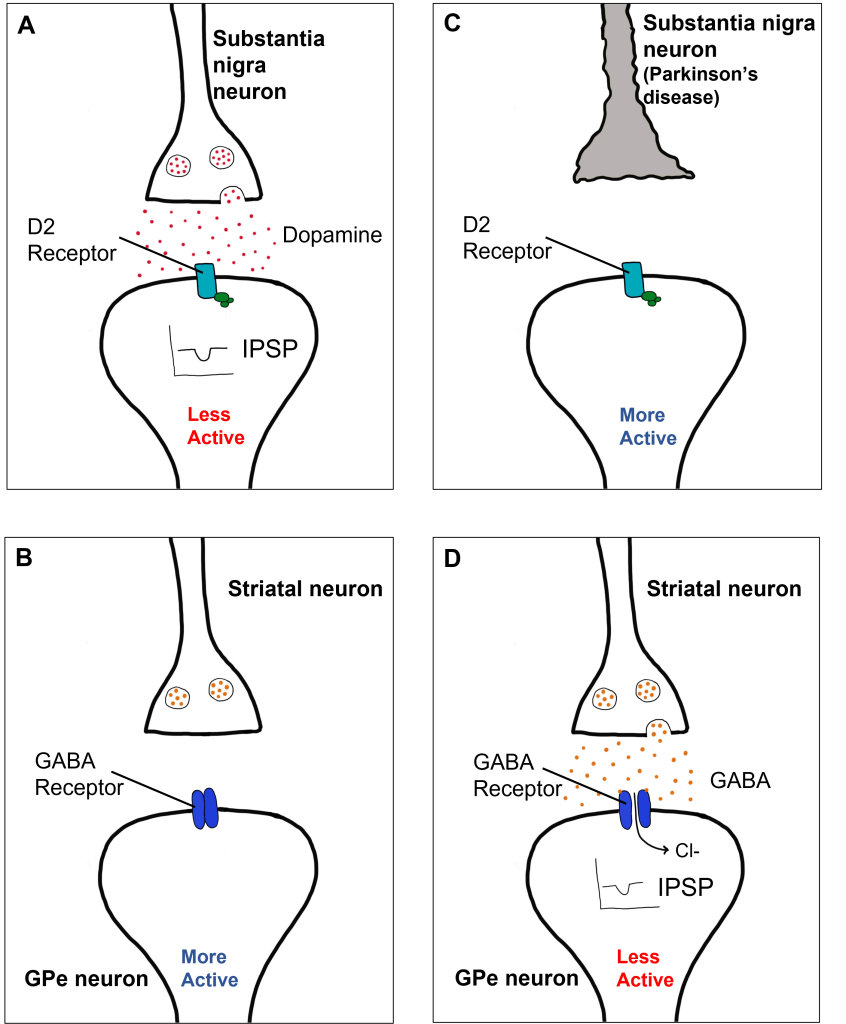
A loss of dopaminergic neurons within the substantia nigra (a midbrain structure) contributes to basal ganglia circuitry disruption in Parkinson’s disease. This loss of substantia nigra cells can be visualized postmortem without the use of histology due to the natural black pigmentation of the substantia nigra cells.
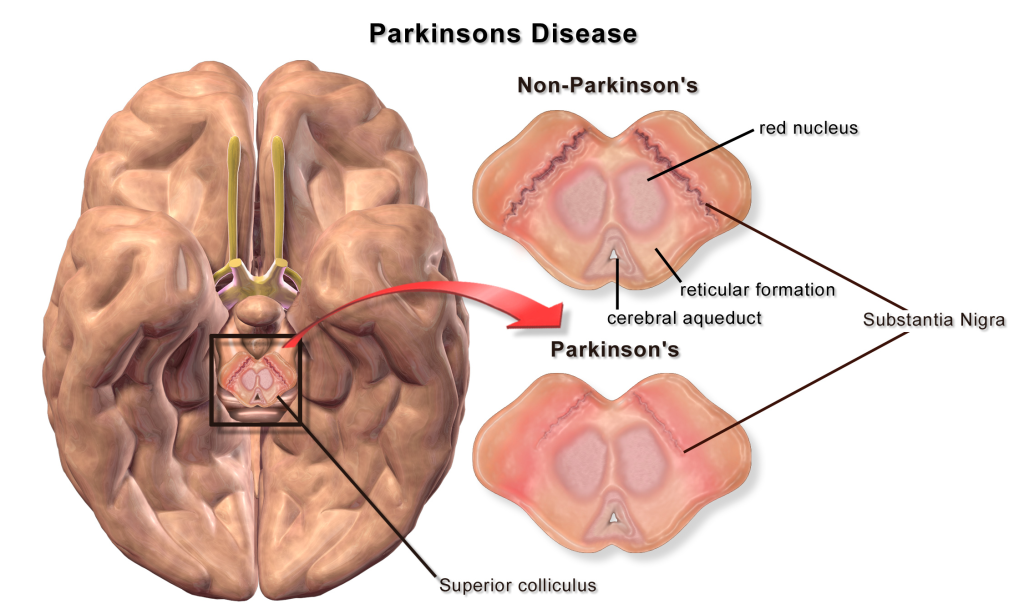
When the substantia nigra cells die, they are no longer able to release dopamine at the striatum appropriately. When less dopamine is released into the striatum, the two populations of striatal neurons are affected differently dependent on the type of dopamine receptor that they express. Recall the basal ganglia circuitry from the previous chapter.
Direct Pathway Dysfunction
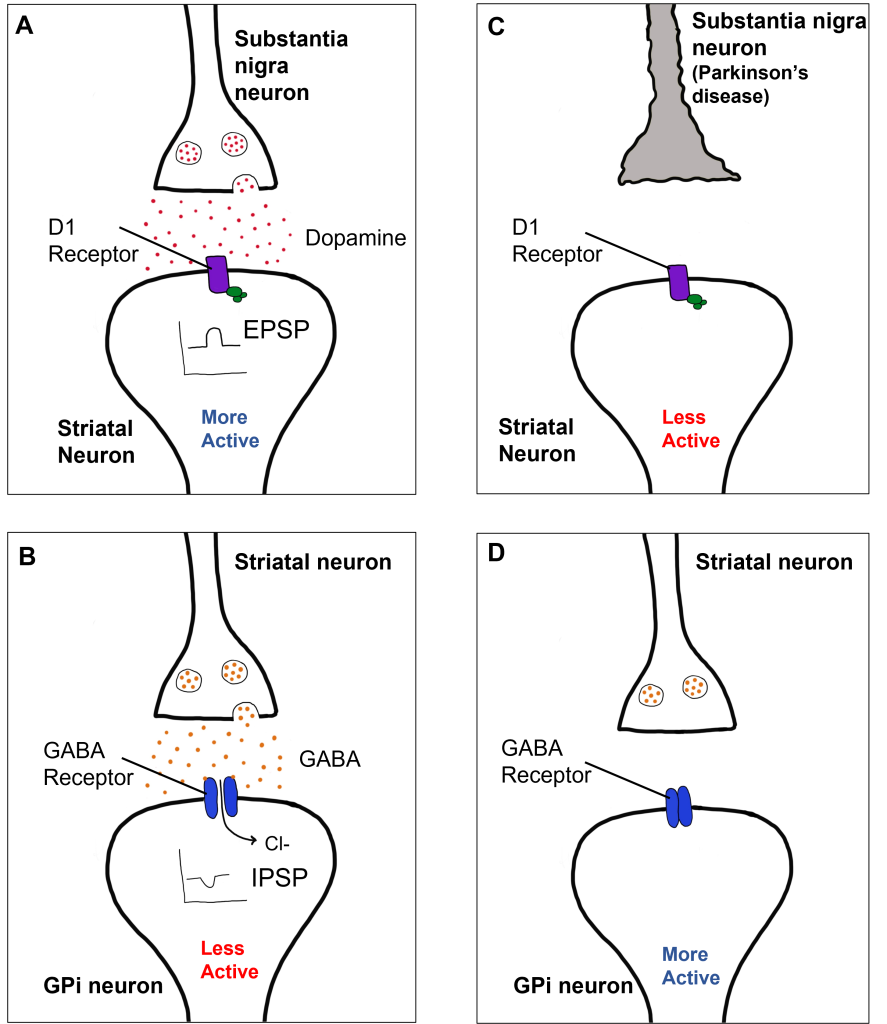
Within the direct pathway, striatal cells (within the caudate) express excitatory D1 receptors that project directly to the globus pallidus internal segment. In the direct pathway, typically, dopamine binds to D1 receptors on striatal neurons, increasing caudate activity. These striatal neurons release GABA onto the globus pallidus internal segment (GPi) neurons, causing the GPi neurons to be less active. When the Gpi neurons are less active, they release less GABA onto the thalamic neurons, increasing thalamic neuron activity and the excitatory projections to the motor cortex, thus increasing movement.
In Parkinson’s disease, there is decreased release of dopamine onto the striatal caudate neurons that express D1 receptors, making them less active. Now that the striatal neurons are not as active, they release less GABA onto GPi neurons, disinhibiting them. The GPi is now more active, releasing more GABA onto thalamic neurons. In response, the thalamic neurons are less active and send less excitatory messages to the cortex, thus causing a decrease in movement overall.
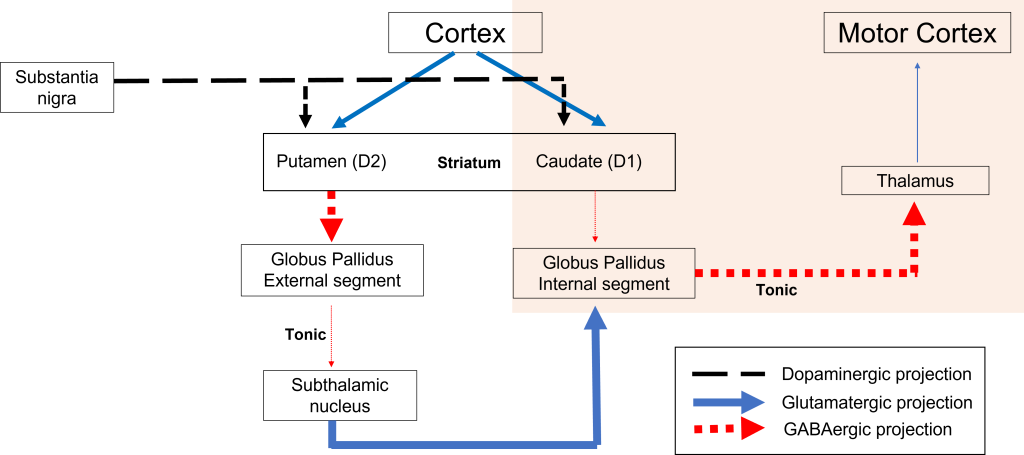
Indirect Pathway Dysfunction
In the indirect pathway, dopamine typically binds to inhibitory D2 receptors on striatal (putamen) neurons, inhibiting striatal neuron activity. These striatal neurons then release less GABA onto the globus pallidus external segment neurons (GPe), causing the GPe neurons to be more active. When the GPe neurons are more active, they release more GABA onto the subthalamic nucleus neurons, decreasing subthalamic neuron activity and the excitatory projections to the GPi. Decreasing activation of the GPi causes the GPi to release less GABA onto the thalamus, which then causes more activation of the motor cortex, thus increasing movement.
In Parkinson’s disease, there is decreased release of dopamine onto the striatal neurons that express D2 receptors, making them more active. Now that the striatal neurons are more active, they release more GABA onto GPe neurons. This inhibits the GPe neurons, causing them to release less GABA onto subthalamic nucleus neurons, disinhibiting the subthalamic nucleus. Increasing the excitatory inputs from the subthalamic nucleus onto the GPi will cause the GPi to release more GABA onto the thalamus. In response, the thalamic neurons are less active and send less excitatory messages to the cortex, thus causing a decrease in movement overall.
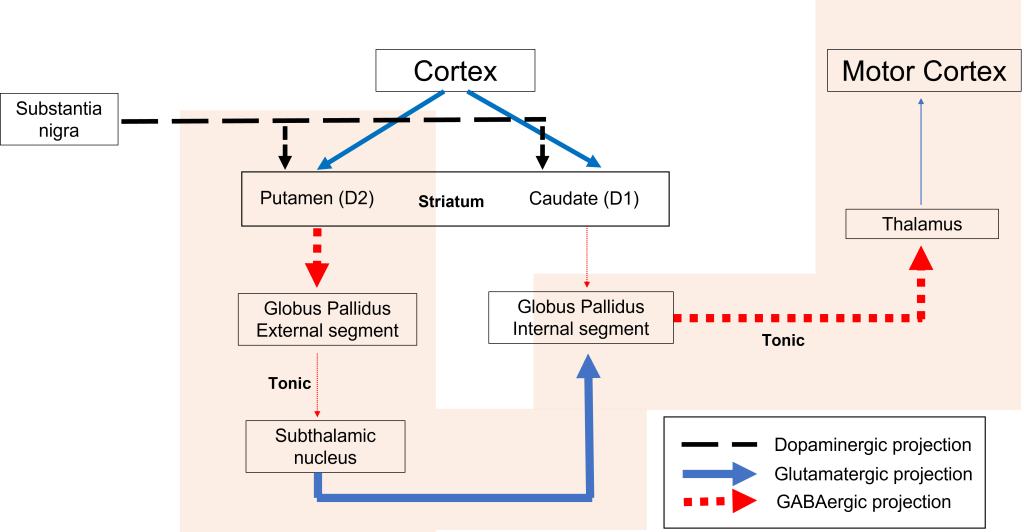
Diagnosis and Treatment
Parkinson’s disease is considered idiopathic because there is not a single known cause. Unfortunately, there are not measurable biomarkers (blood tests, brain scans) to diagnose Parkinson’s disease. Rather, diagnosis of individuals is typically done by a neurologist and a detailed exam. This can be further confirmed by giving medications that are used to treat Parkinson’s disease and determining if symptoms decrease.
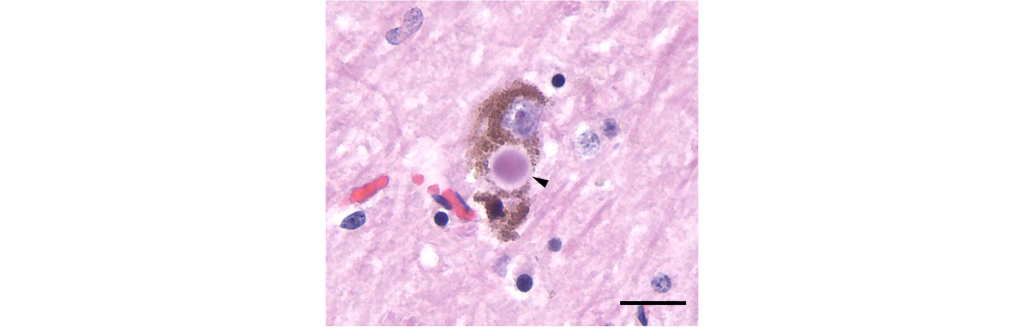
A diagnosis of Parkinson’s disease is confirmed post-mortem through:
- The loss of dopaminergic substantia nigra cells.
- Presence of Lewy bodies.
Lewy bodies are intracellular alpha-nuclein protein aggregates that are believed to potentially displace healthy neurons and lead to the neurodegeneration observed in the disease.
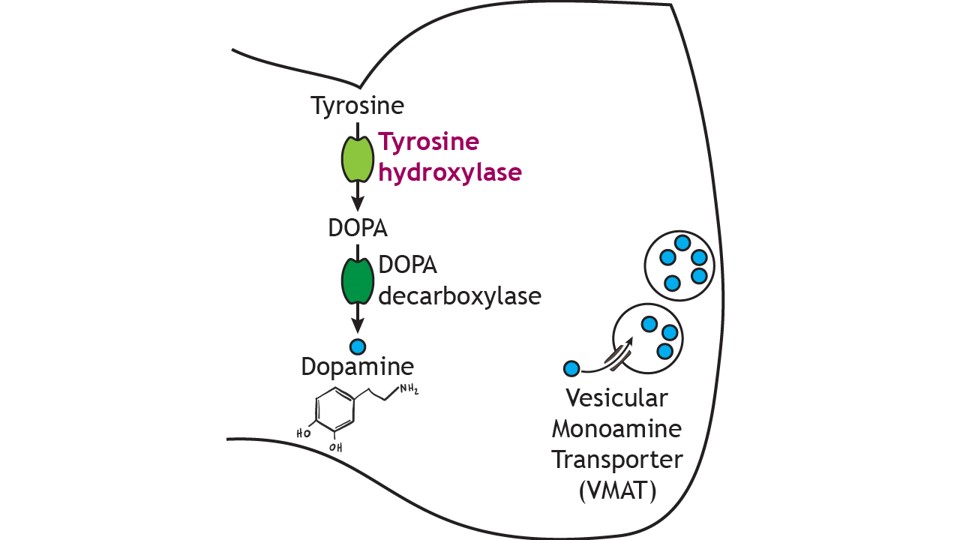
Dopamine cannot be administered as a treatment to those with Parkinson’s disease to replace the loss of dopamine from the substantia nigra due to the inability of dopamine to cross the blood-brain-barrier. Instead, the major drug treatment for the motor symptoms of Parkinson’s disease has been administration of the drug levodopa (L-DOPA). L-DOPA is an intermediate in the synthesis of dopamine. Typically, it is converted into dopamine through the activity of DOPA decarboxylase. It is typically co-administered with other drugs to help prolong its effects. Unfortunately, there are many side effects of L-DOPA including nausea, joint stiffness, dyskinesias (involuntary movements and tics), and psychosis.
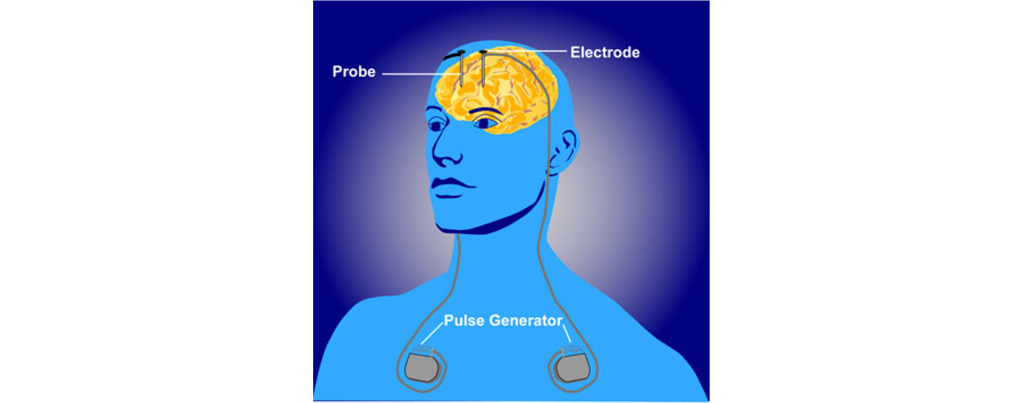
In a medical intervention called deep brain stimulation (DBS), a surgeon implants permanently indwelling electrodes directly into brain tissue. These electrodes are controlled by an external battery pack that delivers preprogrammed stimulation protocols. DBS in the STN is used to alleviate the symptoms of Parkinson’s disease.
Key Takeaways
- Parkinson’s disease is a neurodegenerative disease that causes dopaminergic substantia nigra cells to die, causing dysfunction in the basal ganglia circuitry.
- L-DOPA and deep brain stimulation are treatments for Parkinson’s disease.
Attributions
This chapter is adapted from 38. Neurodegenerative Diseases: Motor in Introduction to Neurobiology by Avinash Singh which is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. Which was adapted from “Neurodegenerative Diseases: Motor” in Introduction to Neuroscience by Valerie Hedges which is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Media Attributions
- Parkinson’s disease Symptoms © Herter, Christian Archibald adapted by Valerie Hedges is licensed under a Public Domain license
- Parkinson’s disease substantia nigra © Bruce Blaus adapted by Valerie Hedges is licensed under a CC BY (Attribution) license
- Parkinson’s diseases effect on D1 receptor function © Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Parkinson’s disease direct pathway Basal Ganglia model © Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- D2 Receptor Effects
- Parkinson’s disease indirect pathway basal ganglia model © Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Lewy Body © Tulemo adapted by Valerie Hedges is licensed under a CC BY-SA (Attribution ShareAlike) license
- dopamine synthesis © Casey Henley adapted by Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Deep brain stimulation © NIMH adapted by Valerie Hedges is licensed under a Public Domain license
Learning Objectives
By the end of this section, you will be able to:
- Discuss how fertilization occurs
- Explain how the embryo forms from the zygote
- Discuss the role of cleavage and gastrulation in animal development
The process in which an organism develops from a single-celled zygote to a multi-cellular organism is complex and well-regulated. The early stages of embryonic development are also crucial for ensuring the fitness of the organism.
Fertilization
Fertilization, pictured in Figure 24.23a is the process in which gametes (an egg and sperm) fuse to form a zygote. The egg and sperm each contain one set of chromosomes. To ensure that the offspring has only one complete diploid set of chromosomes, only one sperm must fuse with one egg. In mammals, the egg is protected by a layer of extracellular matrix consisting mainly of glycoproteins called the zona pellucida. When a sperm binds to the zona pellucida, a series of biochemical events, called the acrosomal reactions, take place. In placental mammals, the acrosome contains digestive enzymes that initiate the degradation of the glycoprotein matrix protecting the egg and allowing the sperm plasma membrane to fuse with the egg plasma membrane, as illustrated in Figure 24.23b. The fusion of these two membranes creates an opening through which the sperm nucleus is transferred into the ovum. The nuclear membranes of the egg and sperm break down and the two haploid genomes condense to form a diploid genome.
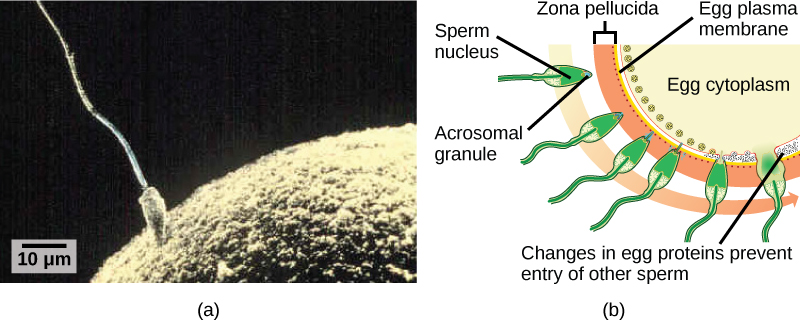
To ensure that no more than one sperm fertilizes the egg, once the acrosomal reactions take place at one location of the egg membrane, the egg releases proteins in other locations to prevent other sperm from fusing with the egg. If this mechanism fails, multiple sperm can fuse with the egg, resulting in polyspermy. The resulting embryo is not genetically viable and dies within a few days.
Cleavage and Blastula Stage
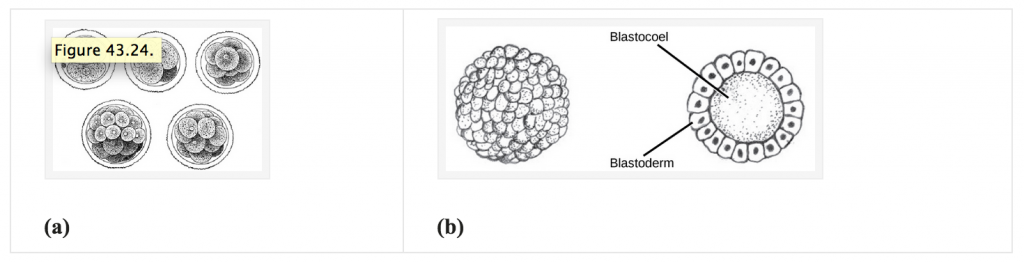
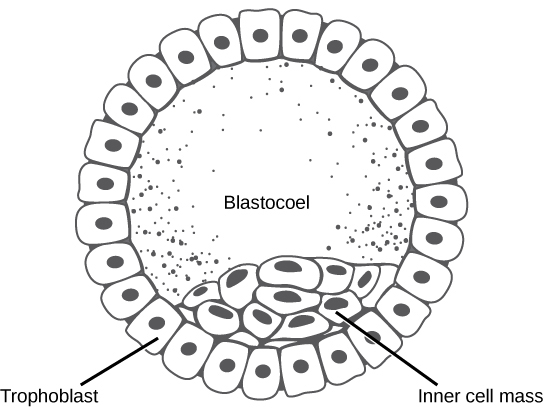
The development of multi-cellular organisms begins from a single-celled zygote, which undergoes rapid cell division to form the blastula. The rapid, multiple rounds of cell division are termed cleavage. Cleavage is illustrated in (Figure 24.24a). After the cleavage has produced over 100 cells, the embryo is called a blastula. The blastula is usually a spherical layer of cells (the blastoderm) surrounding a fluid-filled or yolk-filled cavity (the blastocoel). Mammals at this stage form a structure called the blastocyst, characterized by an inner cell mass that is distinct from the surrounding blastula, shown in Figure 24.24b. During cleavage, the cells divide without an increase in mass; that is, one large single-celled zygote divides into multiple smaller cells. Each cell within the blastula is called a blastomere.
Cleavage can take place in two ways: holoblastic (total) cleavage or meroblastic (partial) cleavage. The type of cleavage depends on the amount of yolk in the eggs. In placental mammals (including humans) where nourishment is provided by the mother’s body, the eggs have a very small amount of yolk and undergo holoblastic cleavage. Other species, such as birds, with a lot of yolk in the egg to nourish the embryo during development, undergo meroblastic cleavage.
In mammals, the blastula forms the blastocyst in the next stage of development. Here the cells in the blastula arrange themselves in two layers: the inner cell mass, and an outer layer called the trophoblast. The inner cell mass is also known as the embryoblast and this mass of cells will go on to form the embryo. At this stage of development, illustrated in Figure 24.25 the inner cell mass consists of embryonic stem cells that will differentiate into the different cell types needed by the organism. The trophoblast will contribute to the placenta and nourish the embryo.
Concept in Action

Visit the Virtual Human Embryo project at the Endowment for Human Development site to step through an interactive that shows the stages of embryo development, including micrographs and rotating 3-D images.
Gastrulation
The typical blastula is a ball of cells. The next stage in embryonic development is the formation of the body plan. The cells in the blastula rearrange themselves spatially to form three layers of cells. This process is called gastrulation. During gastrulation, the blastula folds upon itself to form the three layers of cells. Each of these layers is called a germ layer and each germ layer differentiates into different organ systems.
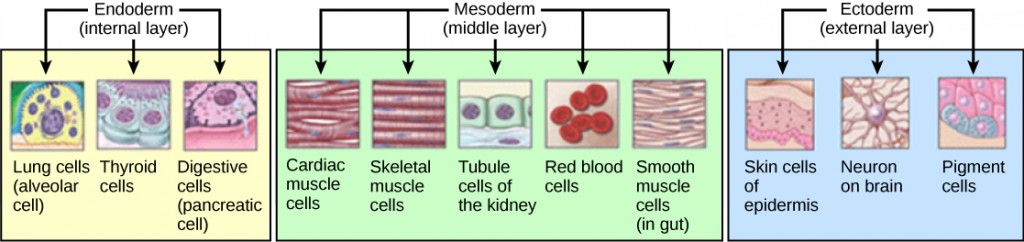
The three germs layers, shown in Figure 24.26, are the endoderm, the ectoderm, and the mesoderm. The ectoderm gives rise to the nervous system and the epidermis. The mesoderm gives rise to the muscle cells and connective tissue in the body. The endoderm gives rise to columnar cells found in the digestive system and many internal organs.
Are Designer Babies in Our Future?
If you could prevent your child from getting a devastating genetic disease, would you do it? Would you select the sex of your child or select for their attractiveness, strength, or intelligence? How far would you go to maximize the possibility of resistance to disease? The genetic engineering of a human child, the production of "designer babies" with desirable phenotypic characteristics, was once a topic restricted to science fiction. This is the case no longer: science fiction is now overlapping into science fact. Many phenotypic choices for offspring are already available, with many more likely to be possible in the not too distant future. Which traits should be selected and how they should be selected are topics of much debate within the worldwide medical community. The ethical and moral line is not always clear or agreed upon, and some fear that modern reproductive technologies could lead to a new form of eugenics.
Eugenics is the use of information and technology from a variety of sources to improve the genetic makeup of the human race. The goal of creating genetically superior humans was quite prevalent (although controversial) in several countries during the early 20th century, but fell into disrepute when Nazi Germany developed an extensive eugenics program in the 1930's and 40's. As part of their program, the Nazis forcibly sterilized hundreds of thousands of the so-called "unfit" and killed tens of thousands of institutionally disabled people as part of a systematic program to develop a genetically superior race of Germans known as Aryans. Ever since, eugenic ideas have not been as publicly expressed, but there are still those who promote them.
Efforts have been made in the past to control traits in human children using donated sperm from men with desired traits. In fact, eugenicist Robert Klark Graham established a sperm bank in 1980 that included samples exclusively from donors with high IQs. The "genius" sperm bank failed to capture the public's imagination and the operation closed in 1999.
In more recent times, the procedure known as prenatal genetic diagnosis (PGD) has been developed. PGD involves the screening of human embryos as part of the process of in vitro fertilization, during which embryos are conceived and grown outside the mother's body for some period of time before they are implanted. The term PGD usually refers to both the diagnosis, selection, and the implantation of the selected embryos.
In the least controversial use of PGD, embryos are tested for the presence of alleles which cause genetic diseases such as sickle cell disease, muscular dystrophy, and hemophilia, in which a single disease-causing allele or pair of alleles has been identified. By excluding embryos containing these alleles from implantation into the mother, the disease is prevented, and the unused embryos are either donated to science or discarded. There are relatively few in the worldwide medical community that question the ethics of this type of procedure, which allows individuals scared to have children because of the alleles they carry to do so successfully. The major limitation to this procedure is its expense. Not usually covered by medical insurance and thus out of reach financially for most couples, only a very small percentage of all live births use such complicated methodologies. Yet, even in cases like these where the ethical issues may seem to be clear-cut, not everyone agrees with the morality of these types of procedures. For example, to those who take the position that human life begins at conception, the discarding of unused embryos, a necessary result of PGD, is unacceptable under any circumstances.
A murkier ethical situation is found in the selection of a child's sex, which is easily performed by PGD. Currently, countries such as Great Britain have banned the selection of a child's sex for reasons other than preventing sex-linked diseases. Other countries allow the procedure for "family balancing", based on the desire of some parents to have at least one child of each sex. Still others, including the United States, have taken a scattershot approach to regulating these practices, essentially leaving it to the individual practicing physician to decide which practices are acceptable and which are not.
Even murkier are rare instances of disabled parents, such as those with deafness or dwarfism, who select embryos via PGD to ensure that they share their disability. These parents usually cite many positive aspects of their disabilities and associated culture as reasons for their choice, which they see as their moral right. To others, to purposely cause a disability in a child violates the basic medical principle of Primum non nocere, "first, do no harm." This procedure, although not illegal in most countries, demonstrates the complexity of ethical issues associated with choosing genetic traits in offspring.
Where could this process lead? Will this technology become more affordable and how should it be used? With the ability of technology to progress rapidly and unpredictably, a lack of definitive guidelines for the use of reproductive technologies before they arise might make it difficult for legislators to keep pace once they are in fact realized, assuming the process needs any government regulation at all. Other bioethicists argue that we should only deal with technologies that exist now, and not in some uncertain future. They argue that these types of procedures will always be expensive and rare, so the fears of eugenics and "master" races are unfounded and overstated. The debate continues.
Summary
The early stages of embryonic development begin with fertilization. The process of fertilization is tightly controlled to ensure that only one sperm fuses with one egg. After fertilization, the zygote undergoes cleavage to form the blastula. The blastula, which in some species is a hollow ball of cells, undergoes a process called gastrulation, in which the three germ layers form. The ectoderm gives rise to the nervous system and the epidermal skin cells, the mesoderm gives rise to the muscle cells and connective tissue in the body, and the endoderm gives rise to columnar cells and internal organs.
Exercises
- Which of the following is false?
- The endoderm, mesoderm, ectoderm are germ layers.
- The trophoblast is a germ layer.
- The inner cell mass is a source of embryonic stem cells.
- The blastula is often a hollow ball of cells.
- During cleavage, the mass of cells:
- increases
- decreases
- doubles with every cell division
- does not change significantly
- What do you think would happen if multiple sperm fused with one egg?
- Why do mammalian eggs have a small concentration of yolk, while bird and reptile eggs have a large concentration of yolk?
- B
- D
- Multiple sperm can fuse with the egg, resulting in polyspermy. The resulting embryo is not genetically viable and dies within a few days.
- Mammalian eggs do not need a lot of yolk because the developing fetus obtains nutrients from the mother. Other species, in which the fetus develops outside of the mother’s body, such as occurs with birds, require a lot of yolk in the egg to nourish the embryo during development.
Glossary
acrosomal reaction
series of biochemical reactions that the sperm uses to break through the zona pellucida
blastocyst
structure formed when cells in the mammalian blastula separate into an inner and outer layer
gastrulation
process in which the blastula folds over itself to form the three germ layers
holoblastic
complete cleavage; takes place in cells with a small amount of yolk
inner cell mass
inner layer of cells in the blastocyst
meroblastic
partial cleavage; takes place in cells with a large amount of yolk
polyspermy
condition in which one egg is fertilized by multiple sperm
trophoblast
outer layer of cells in the blastocyst
zona pellucida
protective layer of glycoproteins on the mammalian egg
Learning Objectives
By the end of this section, you will be able to:
- Discuss how fertilization occurs
- Explain how the embryo forms from the zygote
- Discuss the role of cleavage and gastrulation in animal development
The process in which an organism develops from a single-celled zygote to a multi-cellular organism is complex and well-regulated. The early stages of embryonic development are also crucial for ensuring the fitness of the organism.
Fertilization
Fertilization, pictured in Figure 24.23a is the process in which gametes (an egg and sperm) fuse to form a zygote. The egg and sperm each contain one set of chromosomes. To ensure that the offspring has only one complete diploid set of chromosomes, only one sperm must fuse with one egg. In mammals, the egg is protected by a layer of extracellular matrix consisting mainly of glycoproteins called the zona pellucida. When a sperm binds to the zona pellucida, a series of biochemical events, called the acrosomal reactions, take place. In placental mammals, the acrosome contains digestive enzymes that initiate the degradation of the glycoprotein matrix protecting the egg and allowing the sperm plasma membrane to fuse with the egg plasma membrane, as illustrated in Figure 24.23b. The fusion of these two membranes creates an opening through which the sperm nucleus is transferred into the ovum. The nuclear membranes of the egg and sperm break down and the two haploid genomes condense to form a diploid genome.

To ensure that no more than one sperm fertilizes the egg, once the acrosomal reactions take place at one location of the egg membrane, the egg releases proteins in other locations to prevent other sperm from fusing with the egg. If this mechanism fails, multiple sperm can fuse with the egg, resulting in polyspermy. The resulting embryo is not genetically viable and dies within a few days.
Cleavage and Blastula Stage
The development of multi-cellular organisms begins from a single-celled zygote, which undergoes rapid cell division to form the blastula. The rapid, multiple rounds of cell division are termed cleavage. Cleavage is illustrated in (Figure 24.24a). After the cleavage has produced over 100 cells, the embryo is called a blastula. The blastula is usually a spherical layer of cells (the blastoderm) surrounding a fluid-filled or yolk-filled cavity (the blastocoel). Mammals at this stage form a structure called the blastocyst, characterized by an inner cell mass that is distinct from the surrounding blastula, shown in Figure 24.24b. During cleavage, the cells divide without an increase in mass; that is, one large single-celled zygote divides into multiple smaller cells. Each cell within the blastula is called a blastomere.

Cleavage can take place in two ways: holoblastic (total) cleavage or meroblastic (partial) cleavage. The type of cleavage depends on the amount of yolk in the eggs. In placental mammals (including humans) where nourishment is provided by the mother’s body, the eggs have a very small amount of yolk and undergo holoblastic cleavage. Other species, such as birds, with a lot of yolk in the egg to nourish the embryo during development, undergo meroblastic cleavage.
In mammals, the blastula forms the blastocyst in the next stage of development. Here the cells in the blastula arrange themselves in two layers: the inner cell mass, and an outer layer called the trophoblast. The inner cell mass is also known as the embryoblast and this mass of cells will go on to form the embryo. At this stage of development, illustrated in Figure 24.25 the inner cell mass consists of embryonic stem cells that will differentiate into the different cell types needed by the organism. The trophoblast will contribute to the placenta and nourish the embryo.

Concept in Action

Visit the Virtual Human Embryo project at the Endowment for Human Development site to step through an interactive that shows the stages of embryo development, including micrographs and rotating 3-D images.
Gastrulation
The typical blastula is a ball of cells. The next stage in embryonic development is the formation of the body plan. The cells in the blastula rearrange themselves spatially to form three layers of cells. This process is called gastrulation. During gastrulation, the blastula folds upon itself to form the three layers of cells. Each of these layers is called a germ layer and each germ layer differentiates into different organ systems.
The three germs layers, shown in Figure 24.26, are the endoderm, the ectoderm, and the mesoderm. The ectoderm gives rise to the nervous system and the epidermis. The mesoderm gives rise to the muscle cells and connective tissue in the body. The endoderm gives rise to columnar cells found in the digestive system and many internal organs.

Are Designer Babies in Our Future?

If you could prevent your child from getting a devastating genetic disease, would you do it? Would you select the sex of your child or select for their attractiveness, strength, or intelligence? How far would you go to maximize the possibility of resistance to disease? The genetic engineering of a human child, the production of "designer babies" with desirable phenotypic characteristics, was once a topic restricted to science fiction. This is the case no longer: science fiction is now overlapping into science fact. Many phenotypic choices for offspring are already available, with many more likely to be possible in the not too distant future. Which traits should be selected and how they should be selected are topics of much debate within the worldwide medical community. The ethical and moral line is not always clear or agreed upon, and some fear that modern reproductive technologies could lead to a new form of eugenics.
Eugenics is the use of information and technology from a variety of sources to improve the genetic makeup of the human race. The goal of creating genetically superior humans was quite prevalent (although controversial) in several countries during the early 20th century, but fell into disrepute when Nazi Germany developed an extensive eugenics program in the 1930's and 40's. As part of their program, the Nazis forcibly sterilized hundreds of thousands of the so-called "unfit" and killed tens of thousands of institutionally disabled people as part of a systematic program to develop a genetically superior race of Germans known as Aryans. Ever since, eugenic ideas have not been as publicly expressed, but there are still those who promote them.
Efforts have been made in the past to control traits in human children using donated sperm from men with desired traits. In fact, eugenicist Robert Klark Graham established a sperm bank in 1980 that included samples exclusively from donors with high IQs. The "genius" sperm bank failed to capture the public's imagination and the operation closed in 1999.
In more recent times, the procedure known as prenatal genetic diagnosis (PGD) has been developed. PGD involves the screening of human embryos as part of the process of in vitro fertilization, during which embryos are conceived and grown outside the mother's body for some period of time before they are implanted. The term PGD usually refers to both the diagnosis, selection, and the implantation of the selected embryos.
In the least controversial use of PGD, embryos are tested for the presence of alleles which cause genetic diseases such as sickle cell disease, muscular dystrophy, and hemophilia, in which a single disease-causing allele or pair of alleles has been identified. By excluding embryos containing these alleles from implantation into the mother, the disease is prevented, and the unused embryos are either donated to science or discarded. There are relatively few in the worldwide medical community that question the ethics of this type of procedure, which allows individuals scared to have children because of the alleles they carry to do so successfully. The major limitation to this procedure is its expense. Not usually covered by medical insurance and thus out of reach financially for most couples, only a very small percentage of all live births use such complicated methodologies. Yet, even in cases like these where the ethical issues may seem to be clear-cut, not everyone agrees with the morality of these types of procedures. For example, to those who take the position that human life begins at conception, the discarding of unused embryos, a necessary result of PGD, is unacceptable under any circumstances.
A murkier ethical situation is found in the selection of a child's sex, which is easily performed by PGD. Currently, countries such as Great Britain have banned the selection of a child's sex for reasons other than preventing sex-linked diseases. Other countries allow the procedure for "family balancing", based on the desire of some parents to have at least one child of each sex. Still others, including the United States, have taken a scattershot approach to regulating these practices, essentially leaving it to the individual practicing physician to decide which practices are acceptable and which are not.
Even murkier are rare instances of disabled parents, such as those with deafness or dwarfism, who select embryos via PGD to ensure that they share their disability. These parents usually cite many positive aspects of their disabilities and associated culture as reasons for their choice, which they see as their moral right. To others, to purposely cause a disability in a child violates the basic medical principle of Primum non nocere, "first, do no harm." This procedure, although not illegal in most countries, demonstrates the complexity of ethical issues associated with choosing genetic traits in offspring.
Where could this process lead? Will this technology become more affordable and how should it be used? With the ability of technology to progress rapidly and unpredictably, a lack of definitive guidelines for the use of reproductive technologies before they arise might make it difficult for legislators to keep pace once they are in fact realized, assuming the process needs any government regulation at all. Other bioethicists argue that we should only deal with technologies that exist now, and not in some uncertain future. They argue that these types of procedures will always be expensive and rare, so the fears of eugenics and "master" races are unfounded and overstated. The debate continues.
Summary
The early stages of embryonic development begin with fertilization. The process of fertilization is tightly controlled to ensure that only one sperm fuses with one egg. After fertilization, the zygote undergoes cleavage to form the blastula. The blastula, which in some species is a hollow ball of cells, undergoes a process called gastrulation, in which the three germ layers form. The ectoderm gives rise to the nervous system and the epidermal skin cells, the mesoderm gives rise to the muscle cells and connective tissue in the body, and the endoderm gives rise to columnar cells and internal organs.
Exercises
- Which of the following is false?
- The endoderm, mesoderm, ectoderm are germ layers.
- The trophoblast is a germ layer.
- The inner cell mass is a source of embryonic stem cells.
- The blastula is often a hollow ball of cells.
- During cleavage, the mass of cells:
- increases
- decreases
- doubles with every cell division
- does not change significantly
- What do you think would happen if multiple sperm fused with one egg?
- Why do mammalian eggs have a small concentration of yolk, while bird and reptile eggs have a large concentration of yolk?
- B
- D
- Multiple sperm can fuse with the egg, resulting in polyspermy. The resulting embryo is not genetically viable and dies within a few days.
- Mammalian eggs do not need a lot of yolk because the developing fetus obtains nutrients from the mother. Other species, in which the fetus develops outside of the mother’s body, such as occurs with birds, require a lot of yolk in the egg to nourish the embryo during development.
Glossary
acrosomal reaction
series of biochemical reactions that the sperm uses to break through the zona pellucida
blastocyst
structure formed when cells in the mammalian blastula separate into an inner and outer layer
gastrulation
process in which the blastula folds over itself to form the three germ layers
holoblastic
complete cleavage; takes place in cells with a small amount of yolk
inner cell mass
inner layer of cells in the blastocyst
meroblastic
partial cleavage; takes place in cells with a large amount of yolk
polyspermy
condition in which one egg is fertilized by multiple sperm
trophoblast
outer layer of cells in the blastocyst
zona pellucida
protective layer of glycoproteins on the mammalian egg