49 Molecular Mechanisms of Memory: Hippocampus
Zooming in beyond the level of anatomy, the substrates of learning can be found at the level of synapses. Synapses change in a phenomenon called plasticity. The word “plasticity” refers to a change in synaptic strength, which may be an increase or a decrease. This change may persist for minutes, hours, days, or in some cases, even a whole lifetime.
When synaptic strength is increased and remains elevated, we call this long-term potentiation (LTP). A prolonged weakness of a synapse is called long-term depression (LTD). In our current limited understanding of plasticity, both phenomena are important for a healthy brain, and neither one is always good or always bad. It is also important to clarify that both excitatory synapses and inhibitory synapses can be subject to either LTP or LTD.
The Hippocampus
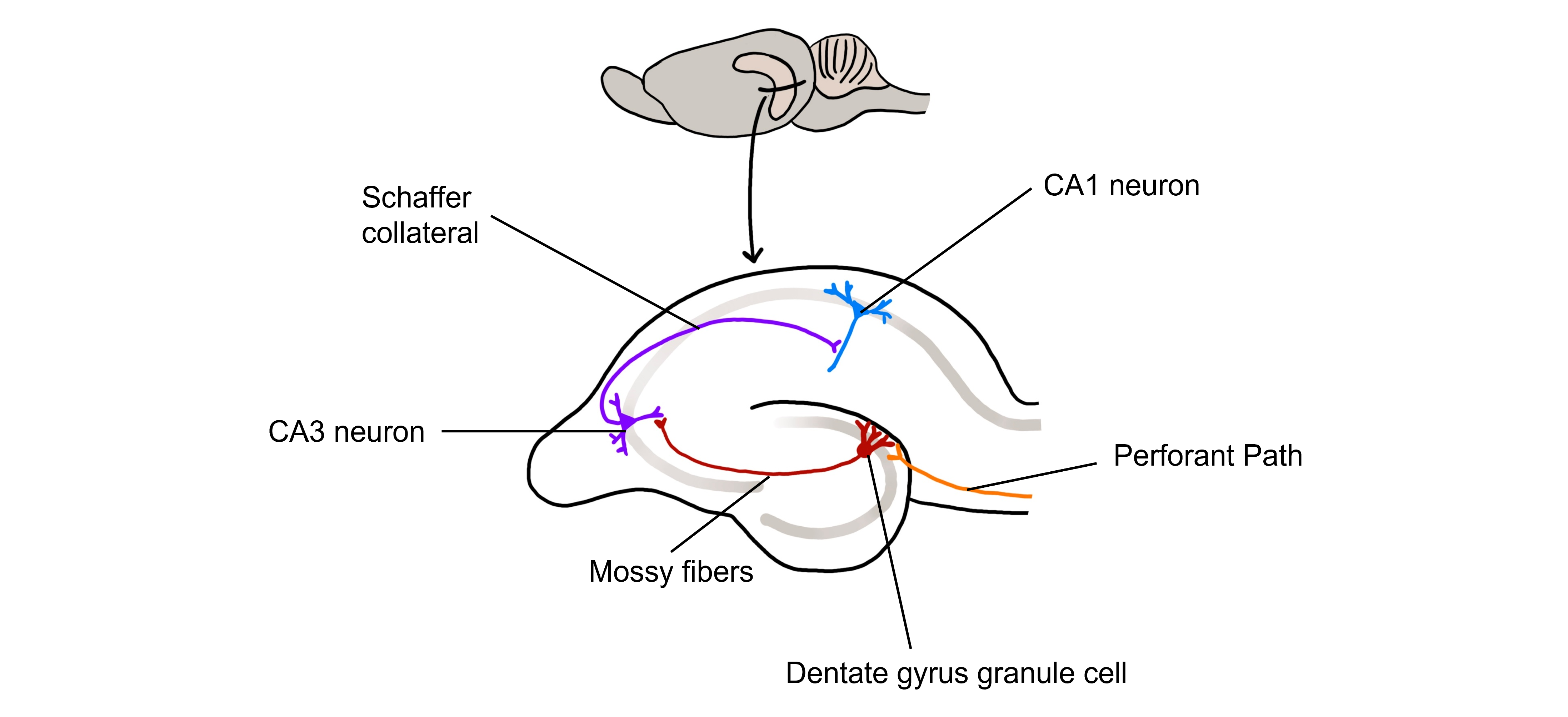
The hippocampus, meaning “seahorse” in Greek, was named based on its morphology. It is located along the ventral and medial surface of the brain. The hippocampus serves as one of the critical structures of the limbic system, a series of subcortical brain structures that are involved in several different complex behaviors, such as emotions and memory. The synaptic connectivity of the hippocampus is very well characterized. Hippocampal synaptic connectivity was first described by Ramon y Cajal, and is made up of three main synaptic connections; sometimes called the trisynaptic circuit.
First, the entorhinal cortex serves as the major input to the hippocampus. This white matter signaling tract is called the perforant pathway, and the neurons synapse onto the granule cells within an area of the hippocampus called the dentate gyrus. The dentate gyrus neurons send their axons, called mossy fibers, to the pyramidal cells of the CA3 region of the hippocampus. The CA3 neurons have axonal projections called Schaffer collaterals that project out of the hippocampus via the fornix and also to neurons within an area of the hippocampus called CA1, which are also neurons that serve as an output of the hippocampus.
Long-Term Potentiation (LTP)
Long-term potentiation (LTP) is a long-lasting increase in synaptic strength, measured by the amplitude of the post-synaptic potential. Plasticity can be measured throughout these connections, but for our purposes we will examine how to create LTP within the Schaffer collateral.
To create LTP within the Schaffer collateral, a brief electrical stimulus must be provided to the presynaptic axons coming from the CA3 cells via a stimulating electrode. The EPSP generated in the postsynaptic CA1 neurons is then measured with a recording electrode to establish a baseline.
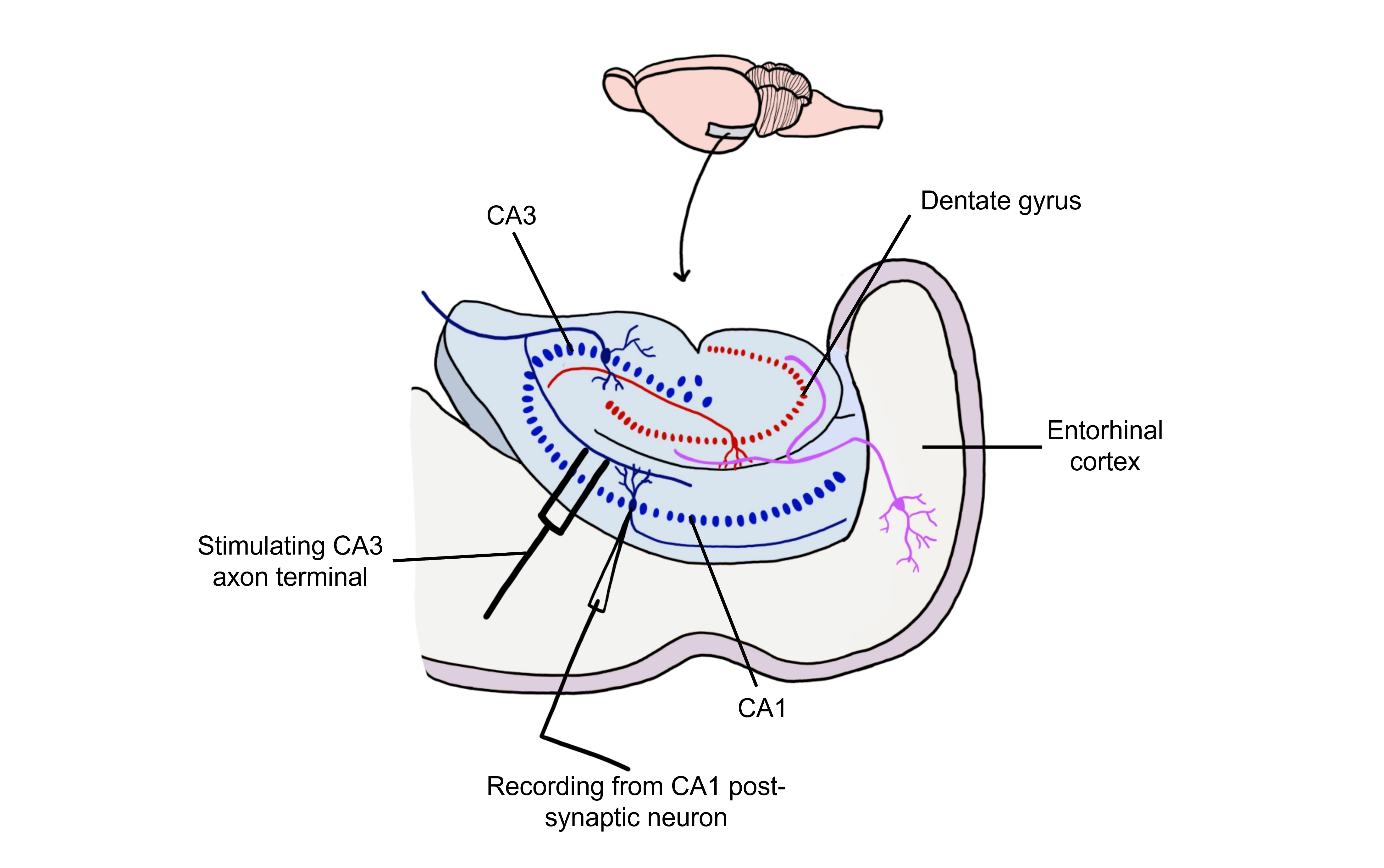
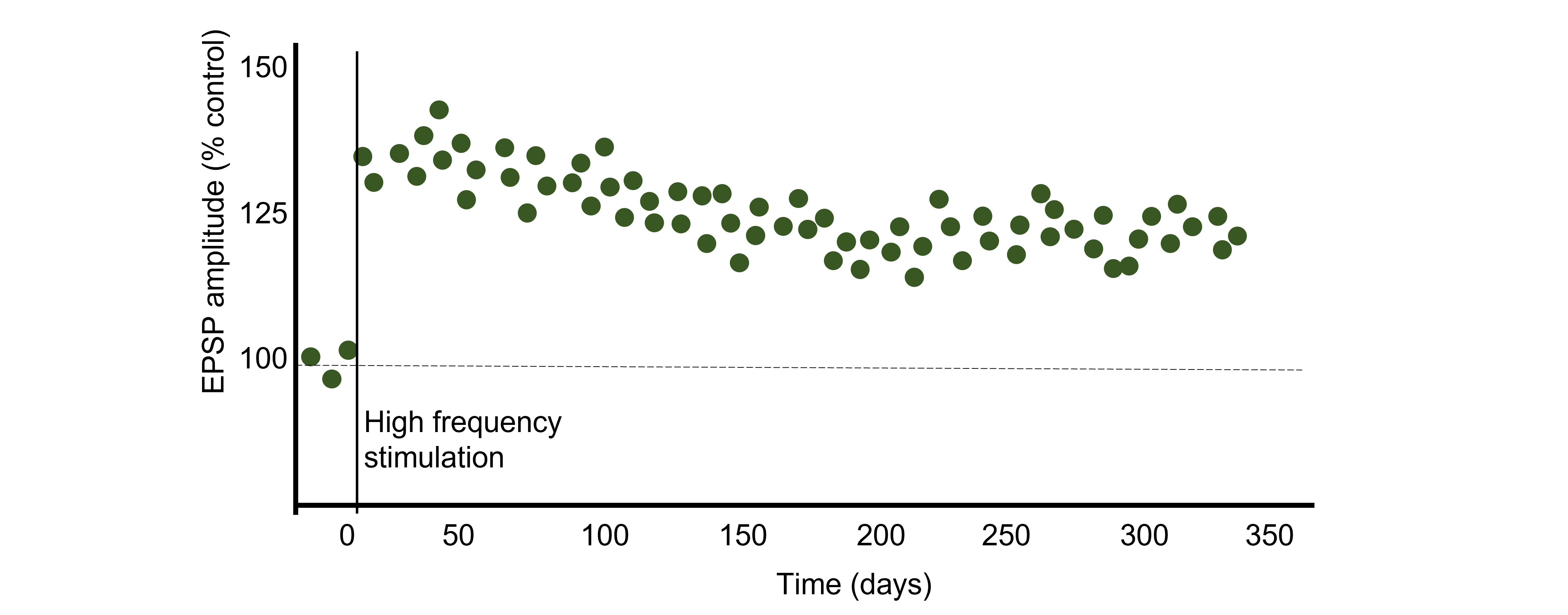
Using Hebb’s theory about plasticity, if “cells that fire together, wire together,” then it stands that repeated stimulation of that synapse would induce a rewiring of the connection, resulting in LTP. To induce LTP within these cells, a tetanus, or a very intense electrical stimulation consisting of 100 stimulations a second (100 Hz) is delivered to the presynaptic CA3 cells for 3 seconds. Following the delivery of tetanus, a single stimulus is provided again and the EPSP is then measured in the postsynaptic CA1 neuron. The delivery of the high frequency stimulation results in an increased amplitude of the postsynaptic EPSP in response to a single stimulus, demonstrating that LTP is a measurable phenomenon.
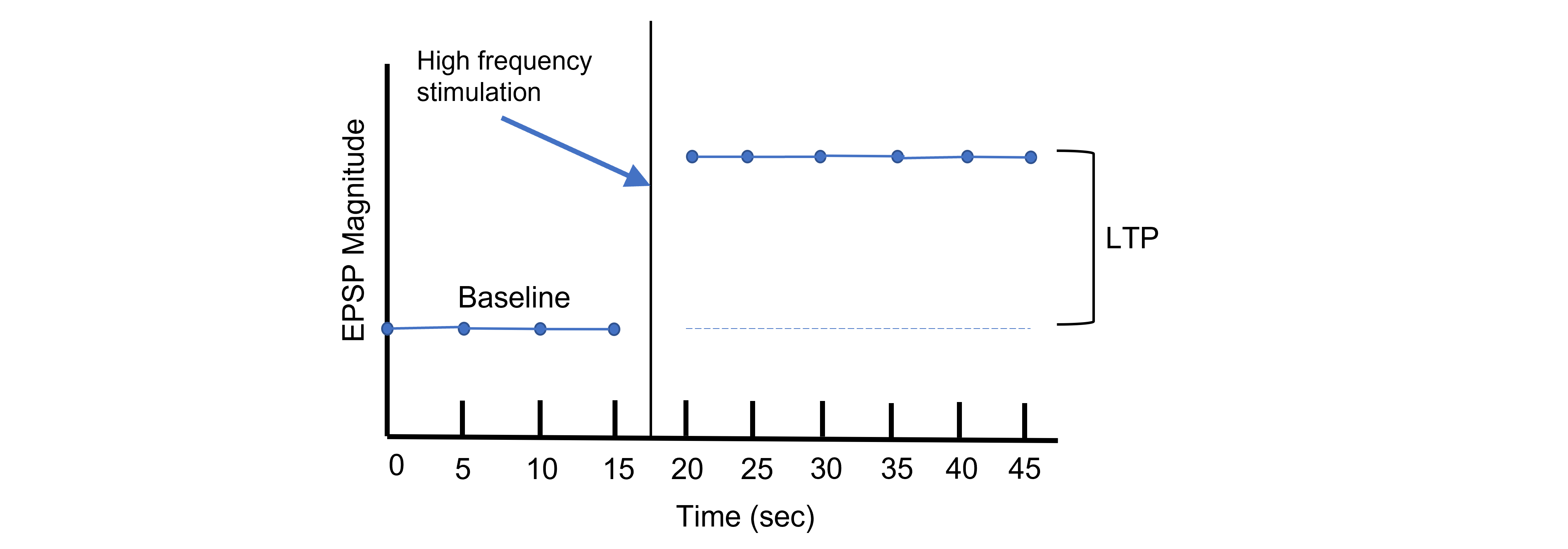
In many cases, LTP is shown graphically by measuring the EPSP amplitude as a percent of the control EPSP amplitude. Prior to tetanus delivery, the amplitude will be measured as 100%, or the same as control. Following tetanus, the amplitude of the EPSP will be larger than it was baseline, for instance 130% indicating a 30% increase in amplitude. Increasing the amplitude of the postsynaptic EPSP increases the likelihood of a neuron firing an action potential by increasing the neuron membrane potential such that it is closer to the threshold potential for the cell.
It has been demonstrated in rodents that the elevated postsynaptic EPSP response is long-lasting and can remain elevated for upwards of one year. In humans, we theorize that some synaptic connections may remain potentiated for our entire lifetime, however investigating this is in humans is ethically constrained.
Glutamate Receptors and LTP
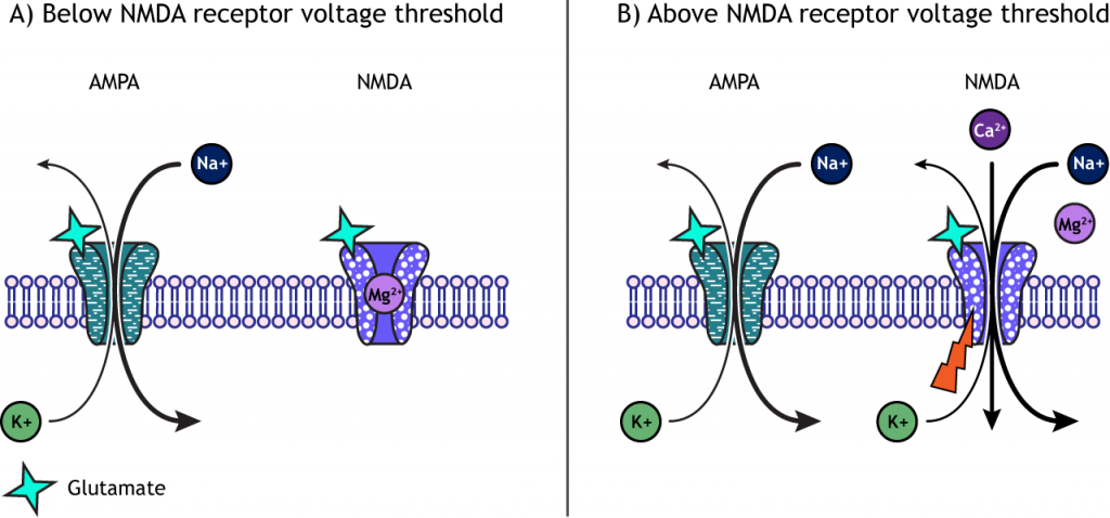
Long-lasting changes in synaptic strength, such as the LTP, are made possible through a series of molecular and cellular level changes. One form of LTP results from a change in the types of glutamatergic receptors. Of the three classes of ionotropic glutamate receptors, two are important for this form of LTP: the AMPA and the NMDA receptors (Chapter 16).
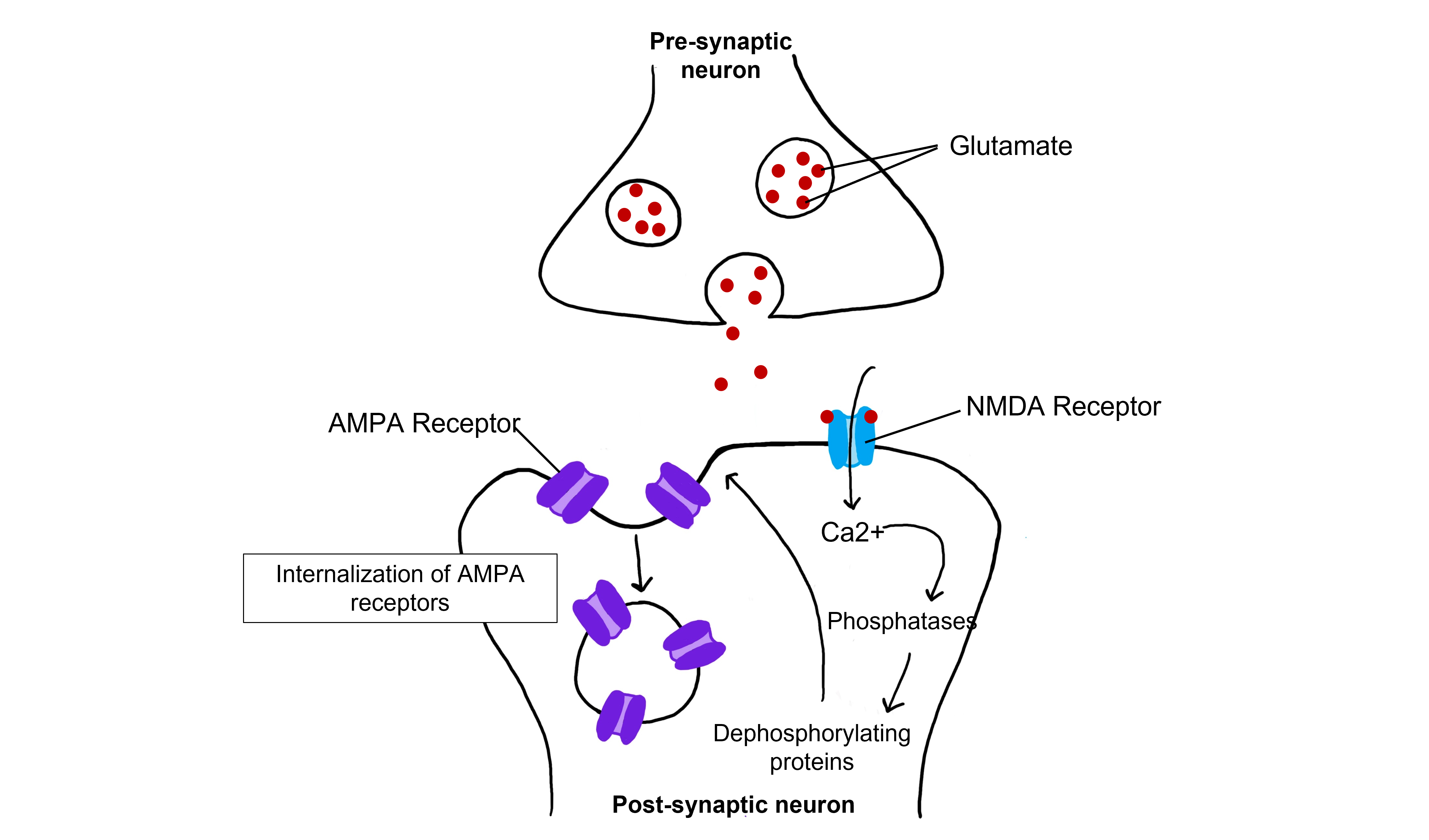
When a molecule of glutamate binds to the active site of the AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptor, the ligand-gated ion channel changes to the open conformation and allows the cations, sodium and potassium, to cross the cell membrane. Sodium moves into the cells more than potassium leaves the cell, leading to depolarization. The NMDA (N-methyl-D-aspartate) receptor requires the binding of glutamate to open, but it is also dependent on voltage. The pore of the NMDA ligand-gated ion channel is blocked by a molecule of magnesium when the membrane potential is below or near resting membrane potential, preventing ions from moving through the channel. Once the cell depolarizes, the magnesium block is expelled from the receptor, which allows sodium, potassium, and calcium to cross the membrane.
The voltage change needed to open the NMDA receptor is usually a result of AMPA receptor activation. Released glutamate binds to both AMPA and NMDA receptors, and sodium influx occurs through open AMPA channels, which depolarizes the cell enough to expel the magnesium ion and allow ion flow through the NMDA receptors.
LTP and Intracellular Calcium
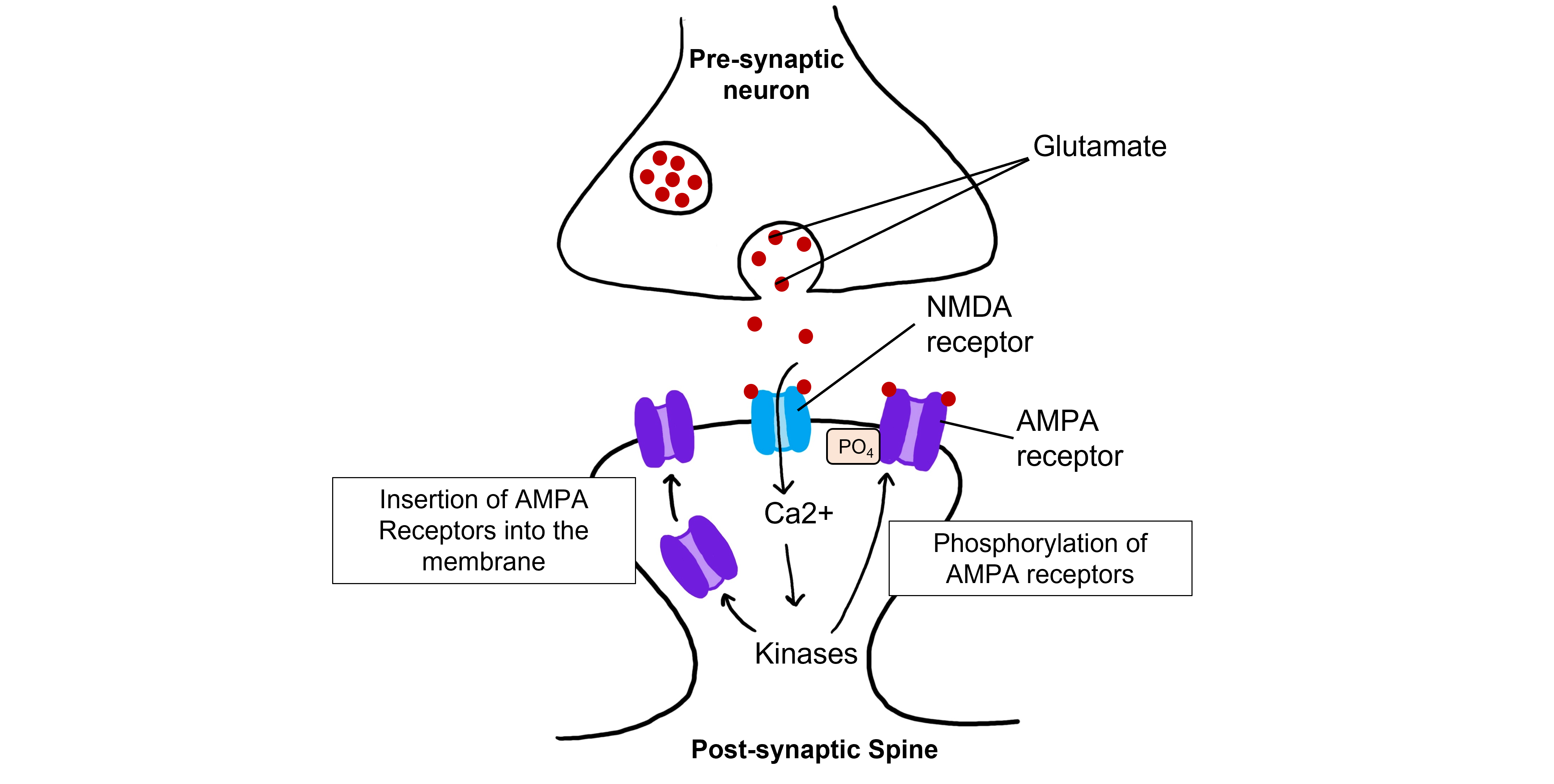
The calcium that enters the cell through the open NMDA receptors activates various kinases including, protein kinase A (PKA), protein kinase C (PKC), and calcium-calmodulin-dependent kinase II (CAMKII). Kinases phosphorylate target proteins within the cell, including AMPA receptors. Phosphorylation of AMPA receptors increases their conductance, allowing more ions to pass through the receptors and leading to a greater degree of depolarization. Further, increased intracellular calcium can lead to the insertion of additional AMPA receptors into the postsynaptic cell membrane, which will also allow more sodium to move into the cell and lead to increased depolarization.
Long-Term Depression (LTD)
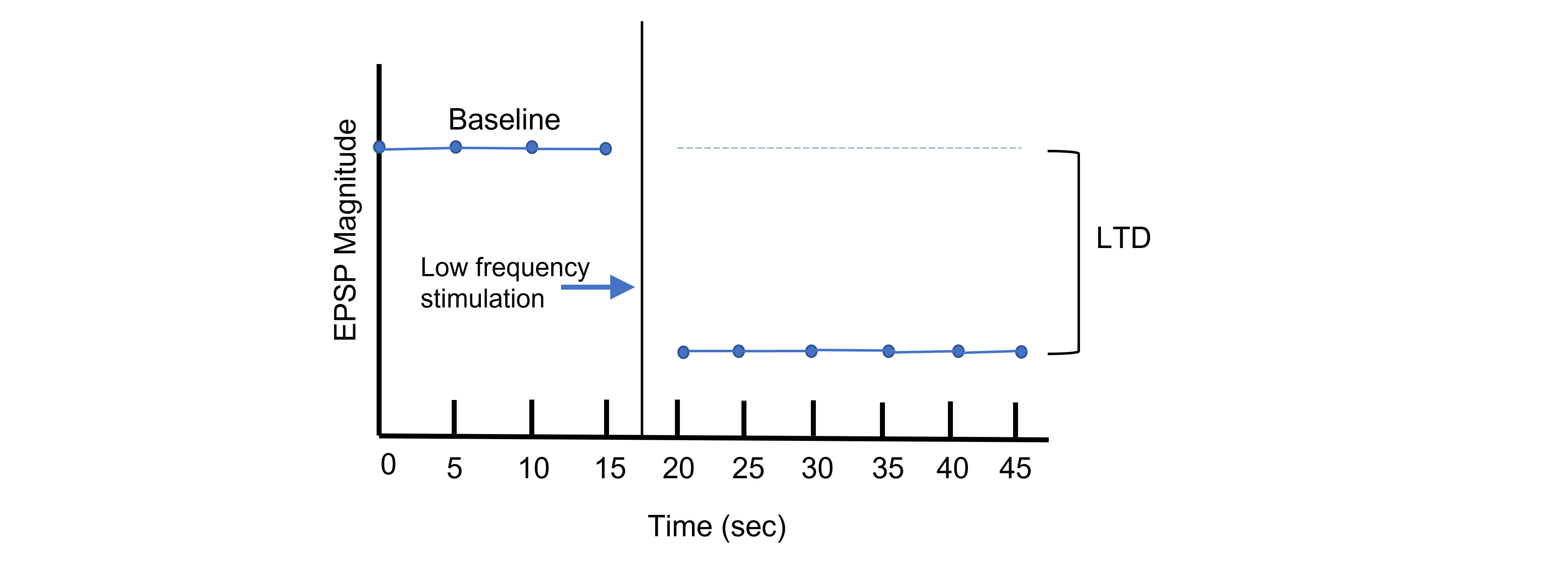
Long-term potentiation increases the strength of synapses. Changes in activity can also cause synapses to be weakened through the process of long-term depression (LTD). Again, using the Schaffer collateral as an example, long-term depression can be induced at the synapses between the CA3 and CA1 neurons by replacing the tetanus stimulation with low-frequency stimulation for longer periods of time. For instance, a 15 min exposure to 1 Hz stimulation will lead to a decreased EPSP amplitude.
Through LTP and LTD, synapses demonstrate bidirectional plasticity that is dependent on the type of stimulation that the synapse receives.
LTD and Intracellular Calcium
Even with low-frequency stimulation of the synapse, glutamate will bind to post-synaptic NMDA receptors and allow for the influx of calcium into the cell. Weak depolarization of the membrane leads to a low amount of calcium entering the cell, which activates a separate class of enzymes call protein phosphatases, specifically protein phosphatase 1 and protein phosphatase 2, that remove phosphate groups from target proteins. Further, LTD causes AMPA receptors to be internalized, or removed from the postsynaptic membrane. Removal of AMPA receptors will decrease excitability.
Relating LTP to Learning
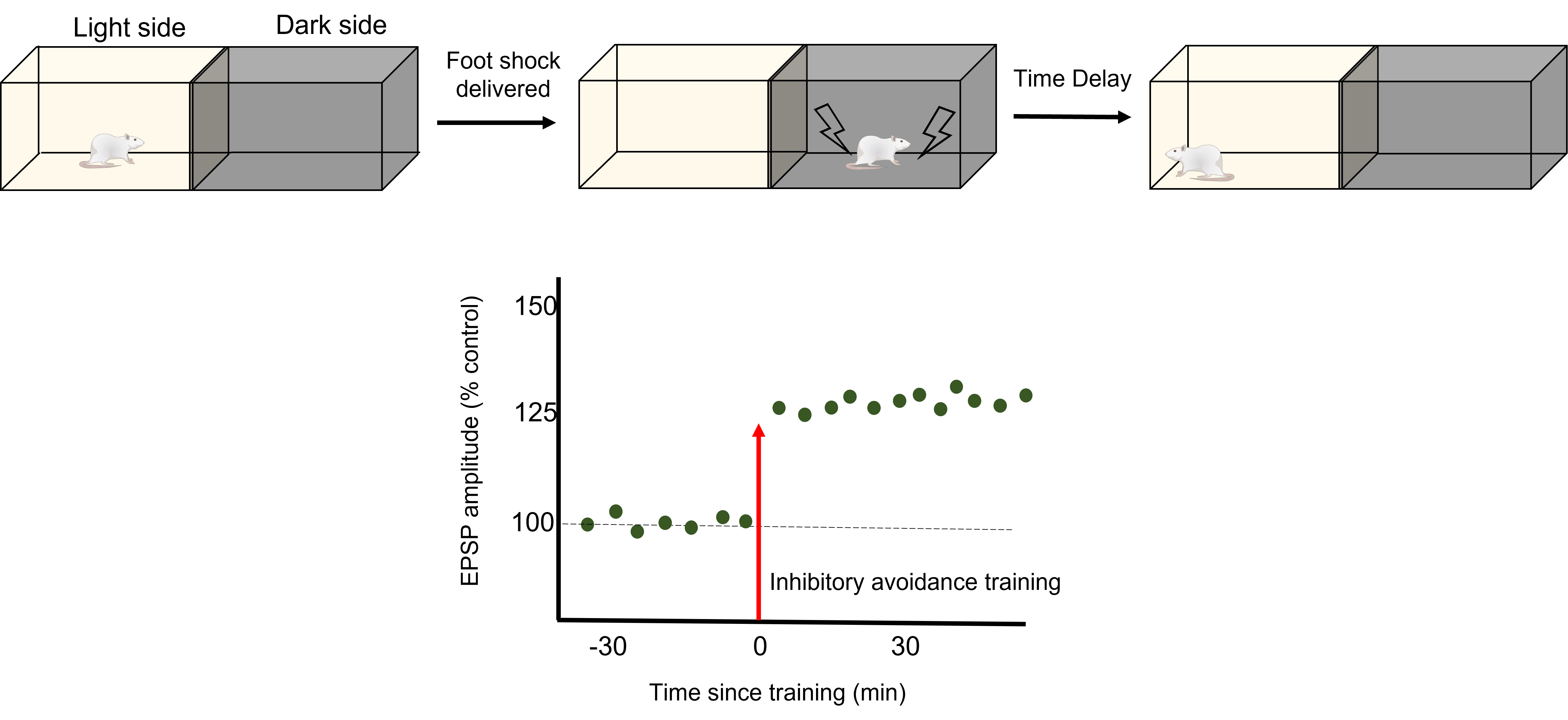
To test how LTP And LTD are directly related to memory, a robust model of learning is used called Inhibitory Avoidance. In an inhibitory avoidance task, a rat learns to associate an environment with an aversive experience. Typically, a two-chamber compartment is used with one light side and one dark side. The animal is permitted to move from the light chamber to the dark chamber, where it receives an electric foot shock. After just one trial with this protocol, the animal will learn to avoid the dark chamber where it received the foot shock.
One trial of inhibitory avoidance led to measurable LTP within the Schaffer collaterals, demonstrating that the behavioral learning corresponded to LTP during this task. Administration of an NMDA receptor blocker to the hippocampus prior to inhibitory avoidance training results in the animals being unable to learn that the dark chamber leads to a foot shock and inhibition of the corresponding LTP.
Key Takeaways
- Plasticity in the form of LTP and LTD occurs at both inhibitory and excitatory synapses.
- The hippocampus has a trisynaptic circuit of connections.
- Long-term potentiation (LTP) is a long-term strengthening of a synapse, whereas long-term depression (LTD) is a long-term weakening of a synapse.
- LTP is demonstrated by observing an increased EPSP amplitude following a tetanus.
- LTD is demonstrated by observing a decreased EPSP amplitude following low frequency stimulation.
- AMPA and NMDA glutamate receptors are important in LTP.
- High levels of intracellular calcium from high frequency stimulation activates protein kinases, which leads to increased conductance of AMPA receptors and insertion of AMPA receptors in the cell membrane.
- Low levels of intracellular calcium from low frequency stimulation activates protein phosphatases, which leads to decreased conductance of AMPA receptors and removal of AMPA receptors in the cell membrane.
- Inhibitory avoidance is a robust learning model in which animals only need one trial before learning occurs. It has been show to also induce LTP.
Attributions
This chapter is adapted from “Molecular Mechanisms of Memory: Hippocampus” in Introduction to Neuroscience by Valerie Hedges which is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Media Attributions
- Rodent hippocampus trisynaptic circuit © Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Schaffer collateral LTP © Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- LTP Graph © Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Graphing postsynaptic response as a percent of control © Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- AMPA NMDA © Casey Henley is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Calcium and LTP © Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- LTD graph © Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Calcium and LTD © Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Inhibitory avoidance © Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
When synaptic strength is increased and remains elevated
Prolonged weakness of a synapse.
very intense electrical stimulation
Height of the action potential (from trough to peak)
a specific membrane potential value that is more positive than resting membrane potential. Threshold potential can differ between cells.
an enzyme that phosphorylates targets (adds a phosphate group) that typically activates the target
enzyme responsible for removing phosphate groups from targets, typically inactivating targets