40 Motivated Behavior: Feeding Behavior
Motivated behaviors are voluntary behaviors that individuals find rewarding or pleasurable. Motivation varies in intensity which will correlate to the probability of performing the associated behavior. Certain behaviors or stimuli, like food or sex, are naturally rewarding because they are necessary for the survival of a species; they are adaptive, and the nervous system has evolved to make these behaviors pleasurable. Rewarding stimuli increases brain activation in brain regions that comprise the reward circuit.
Homeostasis
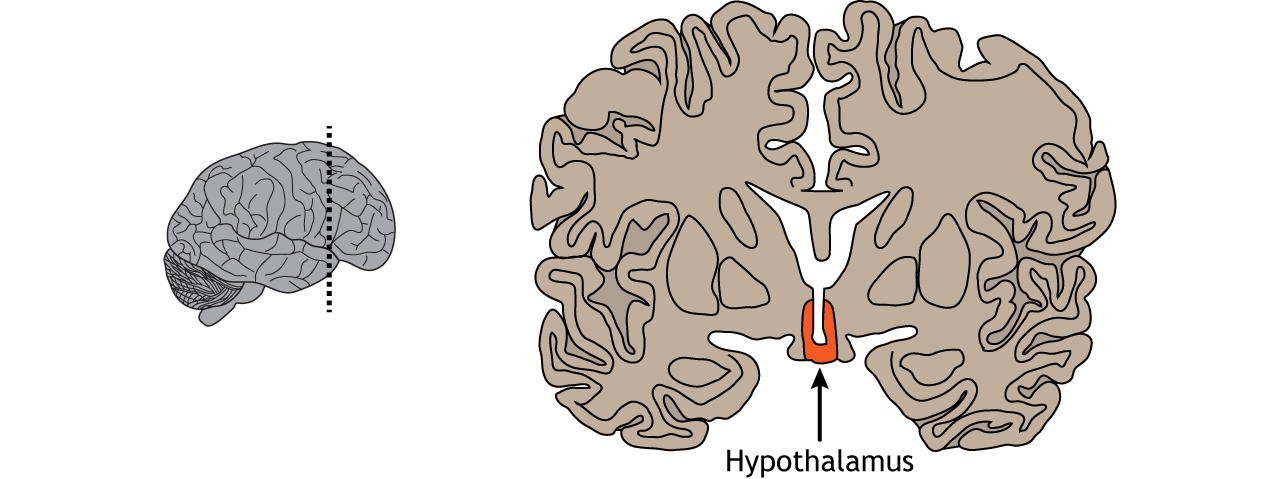
Homeostasis is maintenance of the body’s internal environment within a physiological range. The hypothalamus is important in regulating many homeostatic processes by responding to sensory input when a deviation from homeostatic set point is detected.
Role of Hypothalamus in Homeostasis and Motivated Behavior
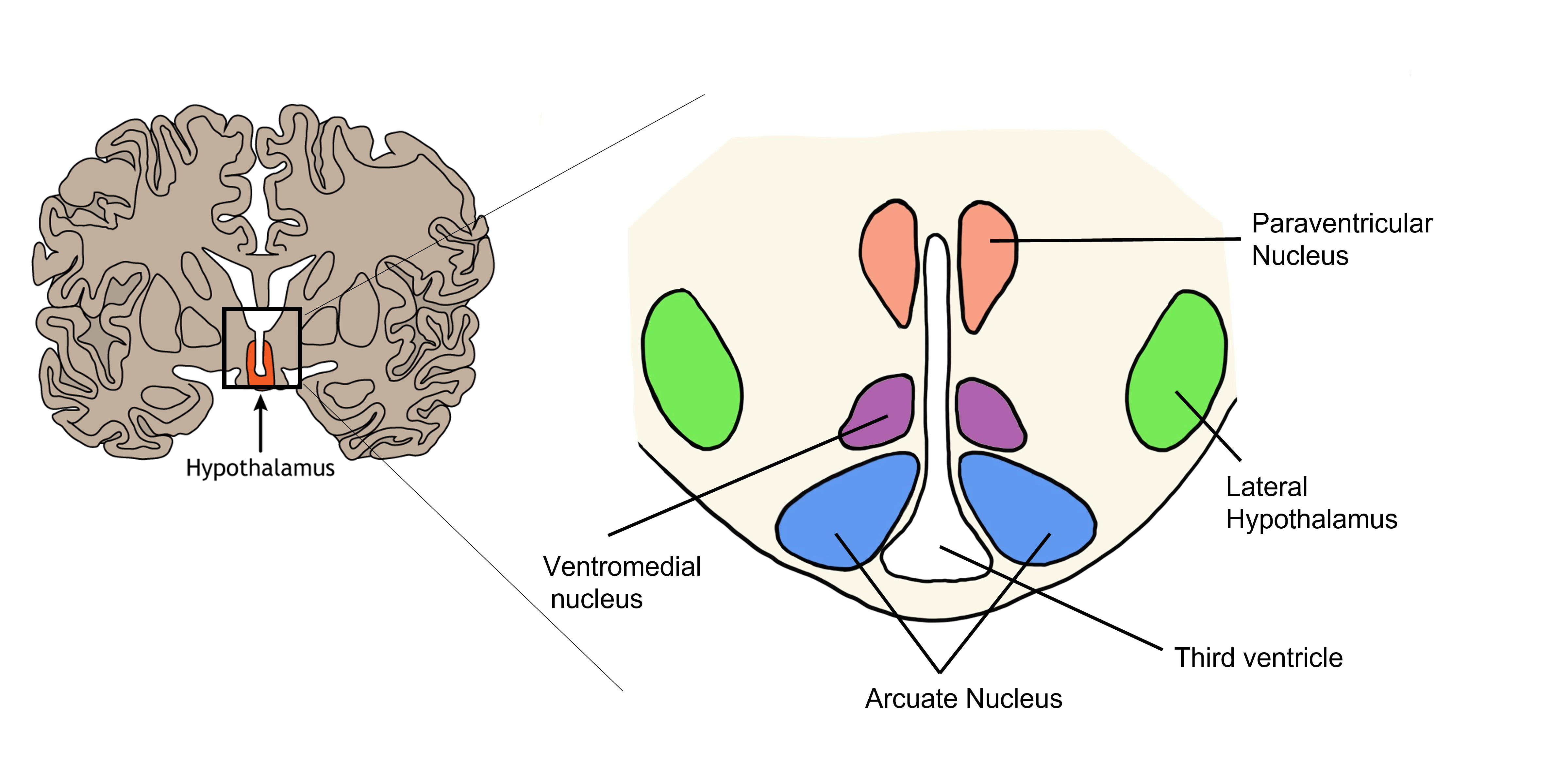
The hypothalamus is located ventrally on the brain and surrounds the third ventricle. Sensory neurons within the hypothalamus measure changes in parameters that need to be regulated within the body, such as temperature, water balance, and feeding. When that parameter is sensed to be outside the normal physiological range, these hypothalamic neurons will initiate a three component process that will act to bring that parameter back within range (restore homeostasis): humoral response, visceromotor response, and somatic motor response.
View the hypothalamus using the BrainFacts.org 3D Brain
Humoral Response
In the humoral response, neurosecretory cells within the hypothalamus regulate release of pituitary hormones into circulation. The word ‘humoral’ comes from the word ‘humor’ which is an older word that refers to different fluids within the body. In the case of the humoral response, the bodily fluid is in reference to the blood where the hormones are being released.
Hypothalamic Control of the Pituitary Gland
The hypothalamus contains two types of neurons that secrete hormones into the pituitary: parvocellular neurosecretory cells and magnocellular neurosecretory cells. Magnocellular neurosecretory cells, the larger type of neurosecretory cell compared to parvocellular neurons, send axons from the hypothalamus down to the pituitary stalk where they terminate on capillaries of the general circulation located within the posterior pituitary. Therefore, the hormones that are released by the posterior pituitary gland are actually made within the hypothalamus. For this reason, the posterior pituitary is considered neural tissue, rather than endocrine tissue.
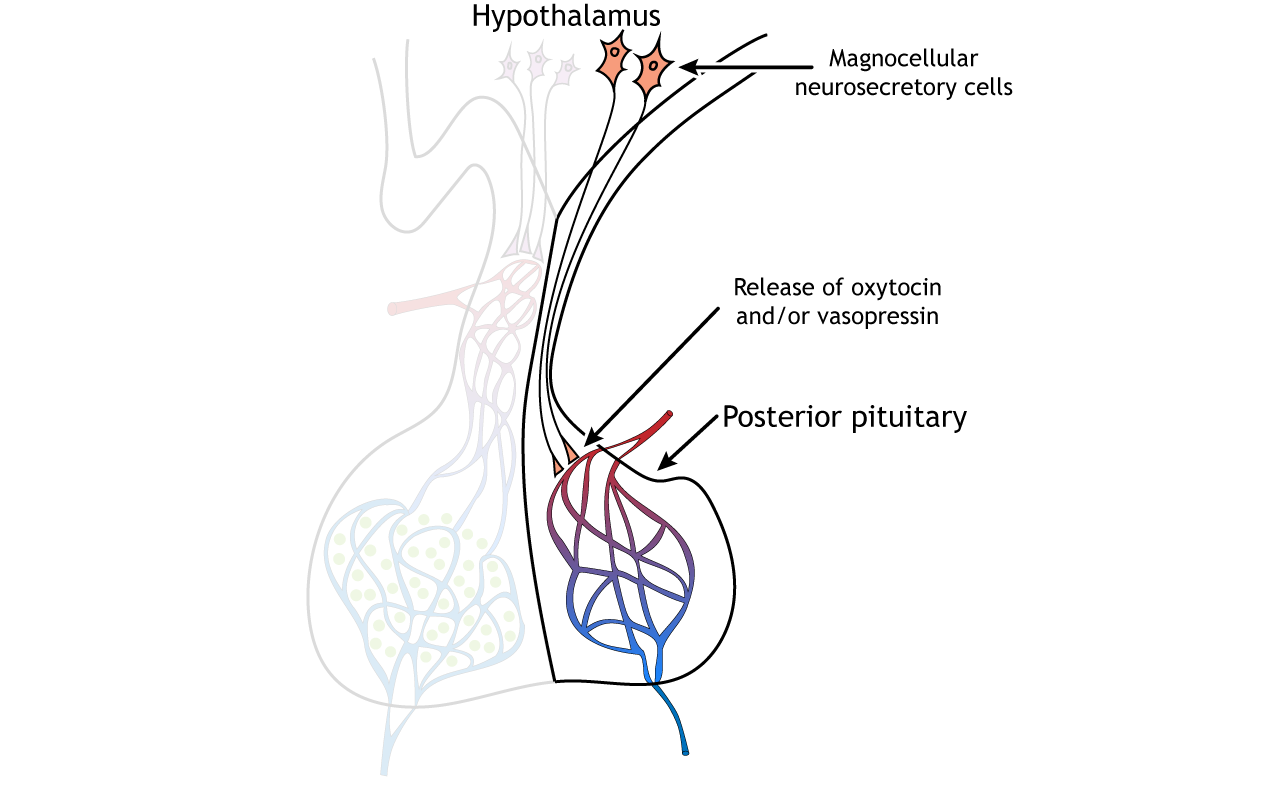
The magnocellular neurons synthesize and release oxytocin and vasopressin, two neuropeptides, into the blood. Oxytocin, often referred to as the love hormone, promotes social bonding. It is released during reproduction and also causes uterine contractions during labor and the milk letdown reflex after birth. Vasopressin, also called antidiuretic hormone, plays a role in regulating salt concentration in the blood by acting on the kidneys to promote water retention and decrease urine production. Vasopressin has also been shown to be involved in bonding, parenting, territoriality, and mate guarding in some animals.
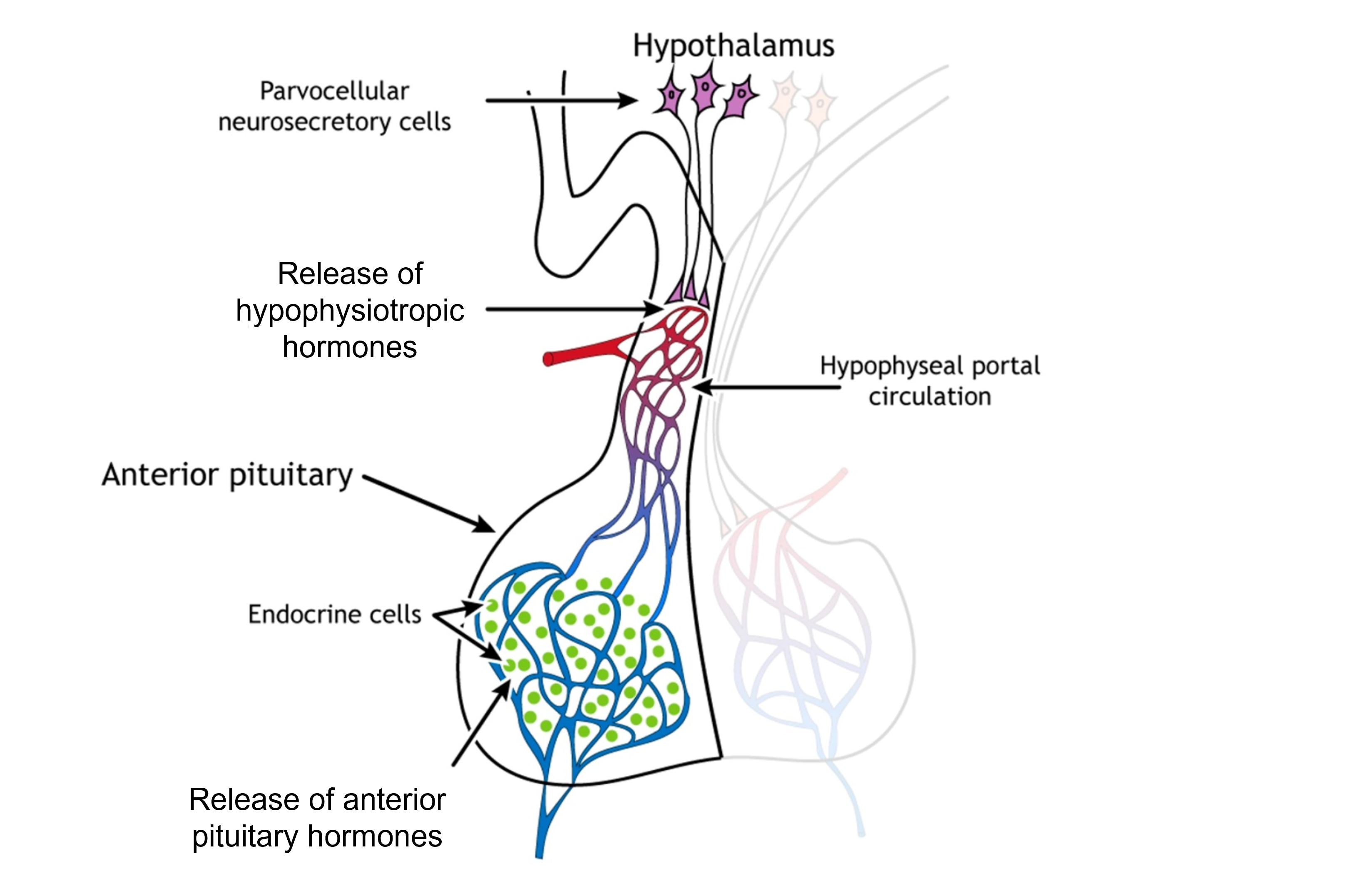
Parvocellular cells are smaller than the magnocellular neurons (parvus means “small” in Latin). These neurons release hypophysiotropic hormones into a specialized capillary system that lies between the hypothalamus and the anterior pituitary gland called the hypophyseal portal circulation. The hypophysiotropic hormones cause anterior pituitary cells to make and release their own hormones. The anterior pituitary gland is therefore endocrine tissue because the cells make their own hormones. There are multiple hormones that are made and released by the anterior pituitary gland. They act on multiple target tissues throughout the body, including the gonads, thyroid glands, adrenal glands, and mammary glands.
Visceromotor Response
The visceromotor response involves hypothalamic neurons that adjust sympathetic and parasympathetic activity to regulate internal organs.
Somatic Motor Response
The somatic motor response involves hypothalamic neurons that trigger an appropriate behavioral response.
Using a drop in temperature as an example, the humoral response will cause the release of hormones into circulation that will allow you to metabolize energy in a different way that will allow for more generation of heat. The visceromotor response would increase sympathetic nervous system activity to ensure that internal organs stay warm. Lastly, the somatic motor response would cause a behavioral change that would result in getting warmer (e.g. getting a coat, moving closer to the fire, or getting out of the cold outside).
Homeostatic Regulation of Body Weight and Food Intake
We obtain energy from our diet and consumption of glucose, which serves as our energy source within the body. The body depends on access to glucose for normal function and survival. To ensure access to glucose when needed, our body stores glucose within the body in fat stores. We are motivated to eat and maintain these body fat stores.
Energy Balance
Body fat stores are regulated after we eat a meal. When we consume more glucose than we can use, the excess glucose is stored within the body as fat stores. Over time, this repeated pattern leads to increased adiposity and can lead to obesity. If energy demands exceed the consumption of glucose, then glucose must be broken down from fat stores and fat is lost. Over time, this leads to decreased adiposity and starvation.
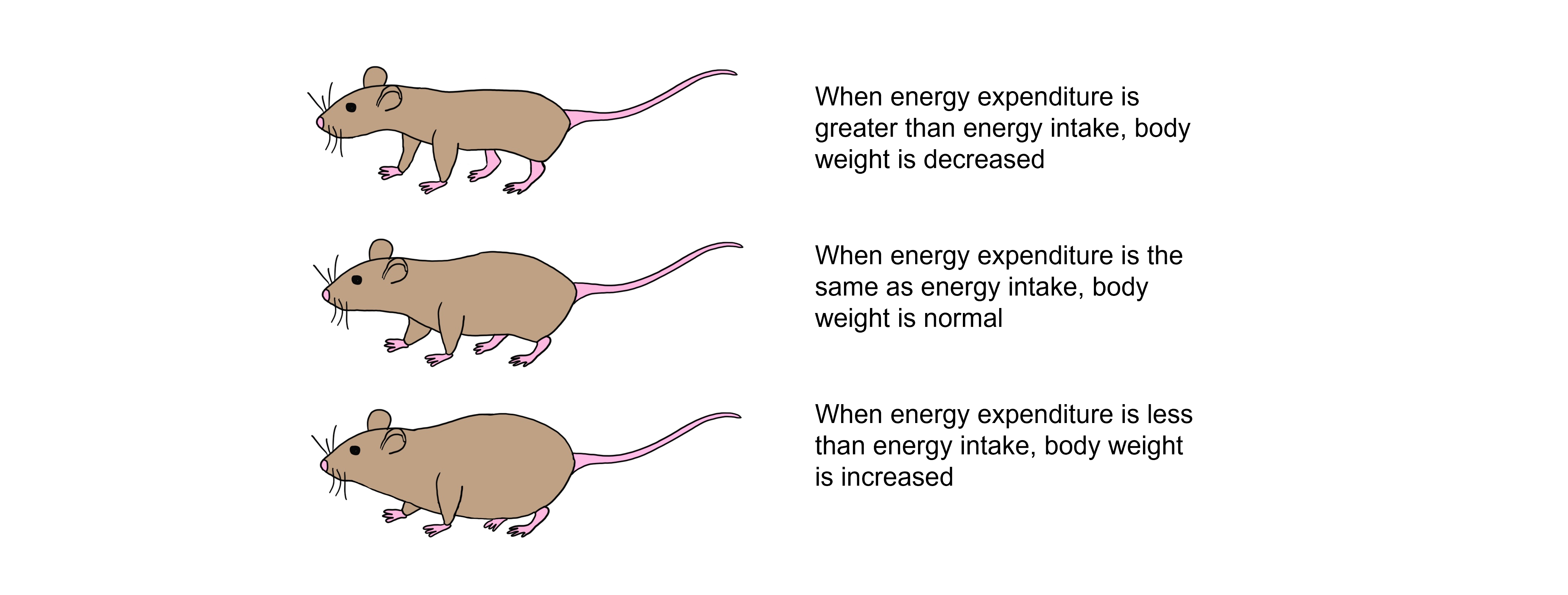
The body tries to defend fat stores and body weight around a particular set point. When rodents have restricted access to calories they experience a decrease in body weight, however when food is freely available again, they gain weight back to their weight prior to calorie restriction. Conversely, animals that are force fed to consume more calories than normal will gain weight, but will lose that gained weight when they are free to regulate their own feeding.
To maintain a balance of fat stores and expended energy, we regulate our feeding behavior. There are both short-term and long-term mechanisms that contribute to feeding regulation and maintenance of fat stores.
Long-Term Regulation of Feeding Behavior
Leptin
The level of fat within the body must somehow be communicated to the brain in a way that ultimately controls feeding behavior. The discovery of genetically obese mice by Dr. Douglas Coleman and colleagues helped determine the source of this signal. The DNA of the genetically obese mice found that these animals were missing both copies of the ob gene. These mice are referred to as ob / ob mice.
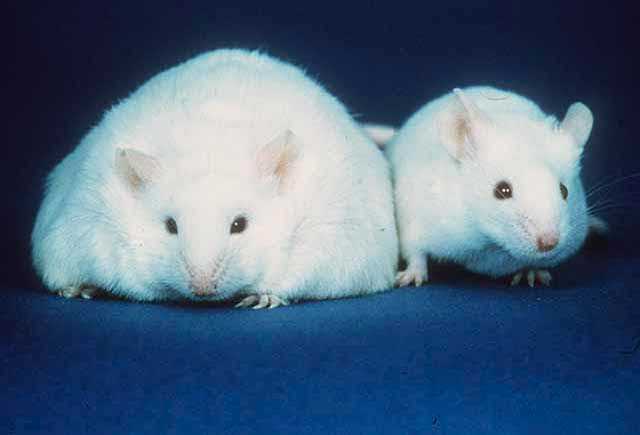
It was theorized that the ob gene must produce a hormone that typically signals that fat reserves are sufficient. Without this gene, and thus without this hormone, the obese mice were not receiving the appropriate signal to stop eating, causing them to over eat and increase fat stores. It was determined that the ob gene codes for the hormone leptin, which is secreted by fat cells. The ob mice had sufficient levels of fat, but did not have the hormone leptin released by the fat cells that would typically decrease feeding behavior. In fact, when ob mice are treated with leptin, they no longer over eat and restore a normal body weight.
More leptin is secreted when fat levels are high. Leptin acts to decrease appetite and increase energy expenditure, which will ultimately decrease levels of fat, and maintain normal fat stores levels in the body. Though it may seem that leptin administration would be an effective treatment for obesity, leptin treatment is only effective for individuals with an identified leptin deficiency. Those without a leptin deficiency have increased levels of leptin due to increased fat stores, but ultimately have neurons with decreased sensitivity to leptin levels.
Role of Hypothalamus in Feeding Behavior
Two areas of the hypothalamus have been historically described with regard to their role in feeding behavior through lesion studies. If the lateral hypothalamus is bilaterally lesioned, it results in lateral hypothalamic syndrome and anorexia, whereas bilateral lesions of the ventromedial hypothalamus results in ventromedial hypothalamic syndrome and overeating/obesity. Though it follows that the lateral hypothalamus causes feeding behavior to increase and the ventromedial hypothalamus causes feeding behavior to decrease, leptin signaling within these structures underlies these behavioral changes.
Arcuate Nucleus
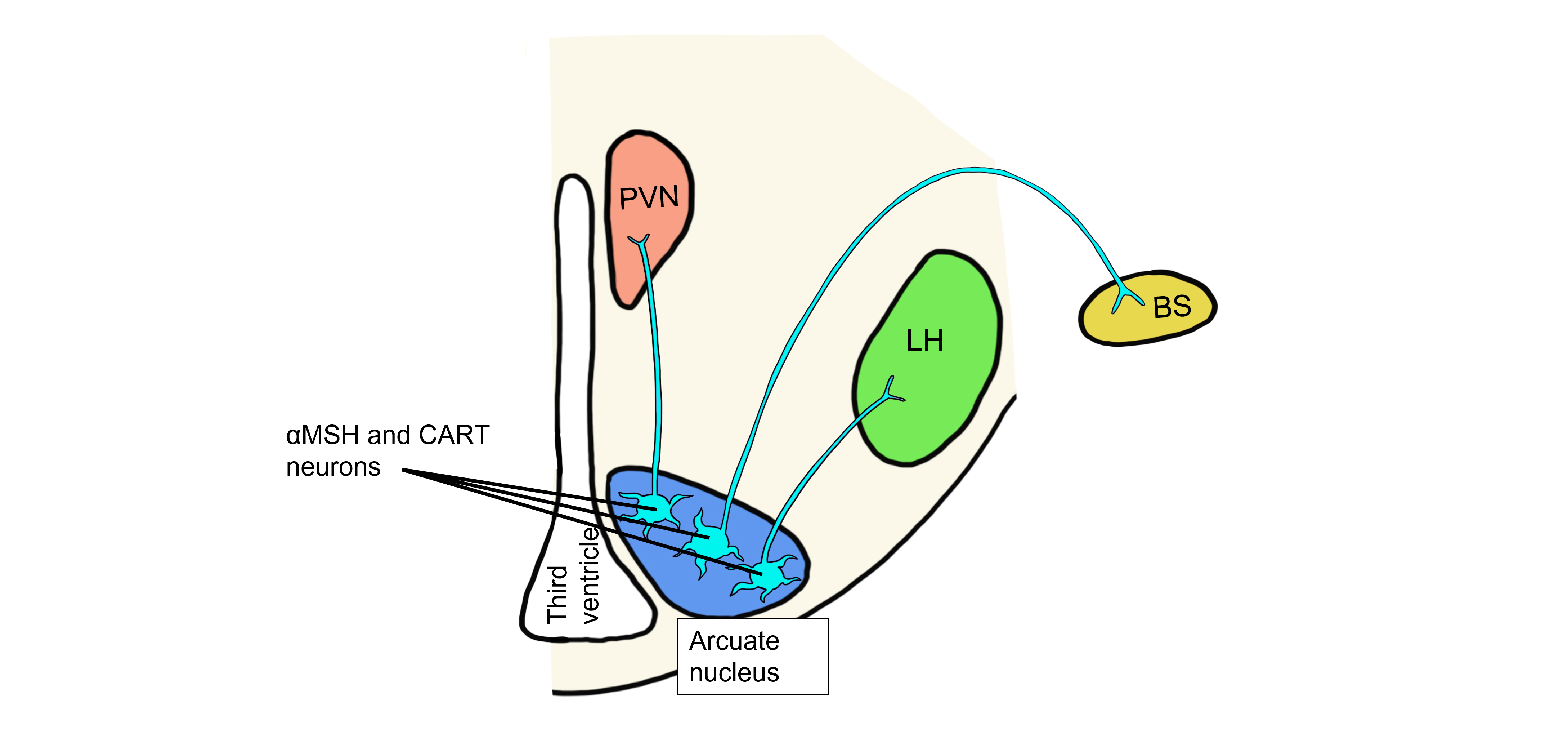
The neurons within the arcuate nucleus of the hypothalamus have receptors that detect levels of circulating leptin. The arcuate nucleus neurons produce peptide neurotransmitters called αMSH (alpha-melanocyte-stimulating hormone) and CART (cocaine- and amphetamine-regulated transcript). Note, that the names of peptides are typically derived from the first identified function of the peptide, but that additional functions are later discovered, which can lead to the names of the peptides being somewhat confusing.
Humoral Response to Increased Leptin
Changes in leptin levels will lead to the humoral, visceromotor, and somatic motor hypothalamic responses.
When fat levels are increased, leptin levels are also increased. In the humoral hypothalamic response, leptin binds to neurons in the arcuate nucleus. This population of arcuate neurons project to the paraventricular nucleus (PVN) and release αMSH and CART onto the PVN neurons. In response to the binding of αMSH and CART, the PVN neurons cause the release of the hypophysiotropic hormones. The anterior pituitary gland will then release thyroid stimulating hormone (TSH) and adrenocorticotropic hormone (ACTH), which will both act to raise the metabolic rate within the body.
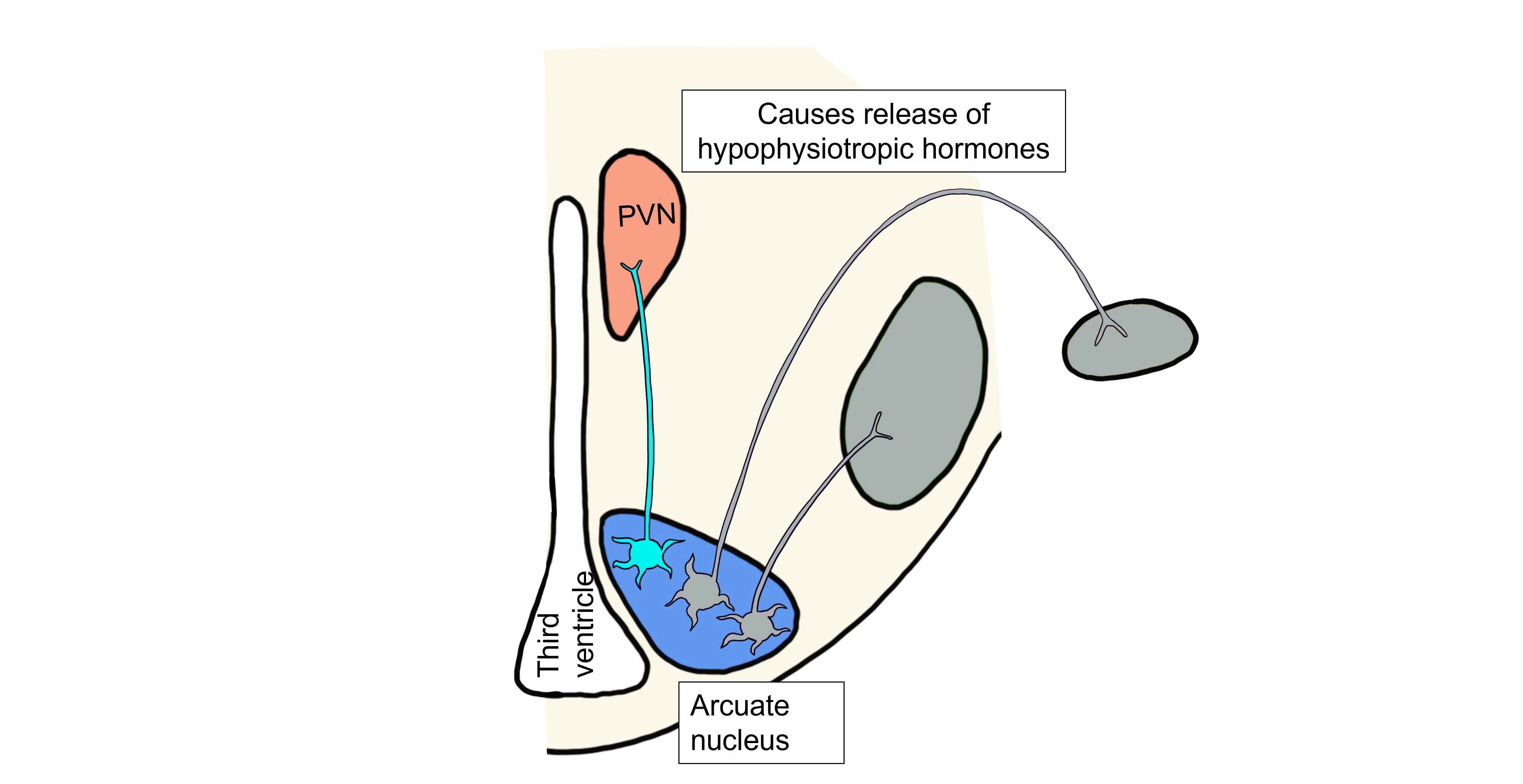
Visceromotor Response to Increased Leptin
When leptin levels are high, leptin will also bind to a separate population of arcuate neurons that project to the brainstem and control the hypothalamic visceromotor response. These arcuate neurons release αMSH and CART onto brainstem neurons that will act to increase sympathetic nervous system activity, raising the metabolic rate and increasing body temperature.
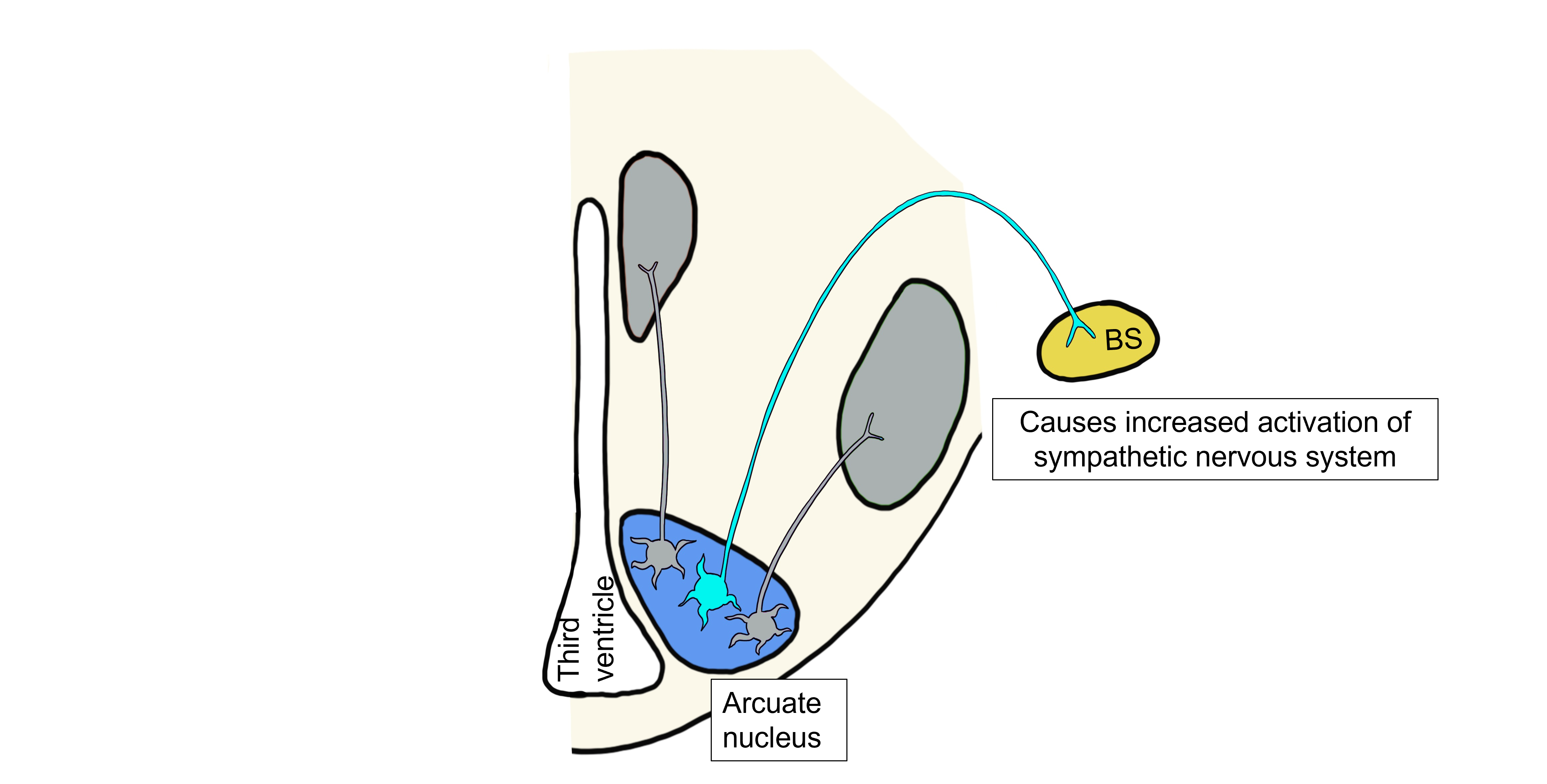
Somatic Motor Response to Increased Leptin
Lastly, when leptin levels are high, leptin binds to a third population of arcuate neurons that project to the lateral hypothalamus and control the hypothalamic somatic motor response. These arcuate neurons release αMSH and CART onto lateral hypothalamus neurons. αMSH and CART inhibit the lateral hypothalamus, inhibiting feeding behavior.
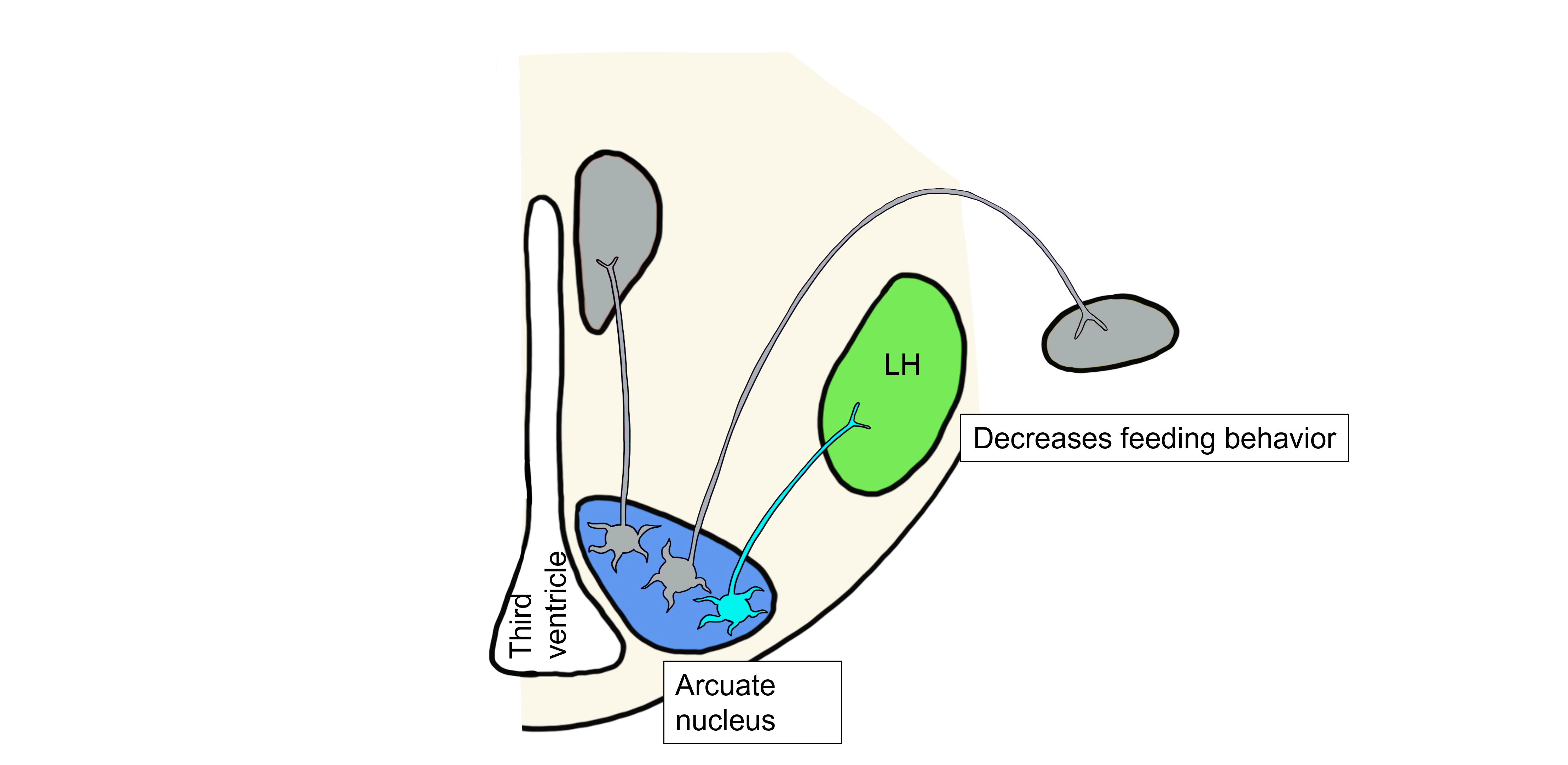
Overall, the increase in metabolic rate, increase in body temperature, and decreased feeding will all ultimately lead to a decrease in body fat and thus a decrease in leptin levels, bringing fat and leptin levels back within the physiological setpoint range. αMSH, CART, and Leptin are all examples of anorexigenic, or satiety, signals. Collectively, anorexigenic signals decrease feeding behavior and decrease body weight in response to increased adiposity.
Humoral Response to Decreased Leptin
When fat levels are decreased, leptin levels are also decreased. When leptin levels drop, an entirely different set of neurons within the arcuate nucleus are activated that release the neuropeptides neuropeptide Y (NPY) and agouti-related peptide (AgRP).
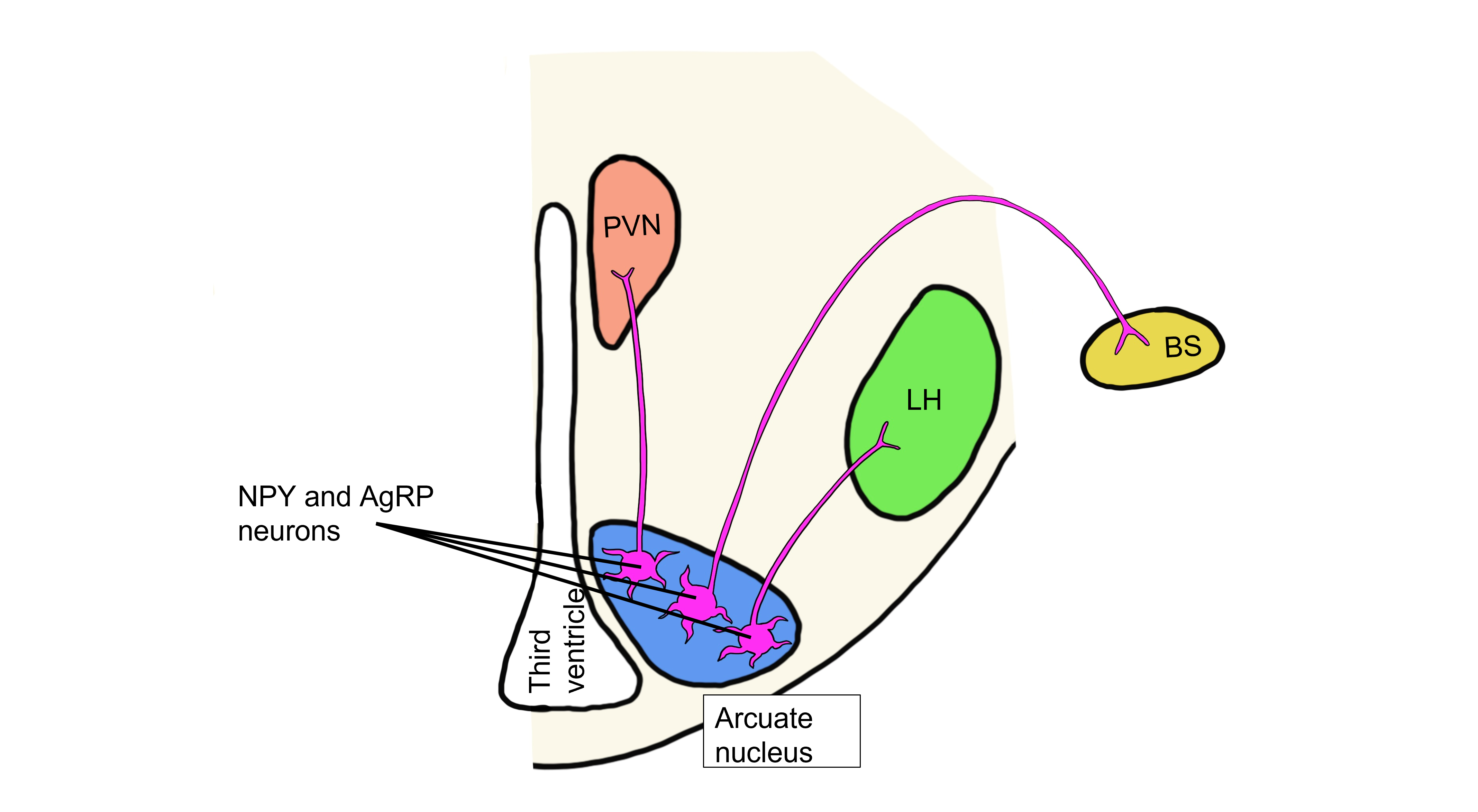
In the humoral hypothalamic response, the drop in leptin activates neurons in the arcuate nucleus. This population of arcuate neurons project to the paraventricular nucleus (PVN) and release NPY and AgRP onto the PVN neurons. These neuropeptides inhibit the PVN, thereby inhibiting release of the hypophysiotropic hormones, and ultimately inhibiting release of TSH and ACTH. As a result, metabolic activity in the body is decreased.
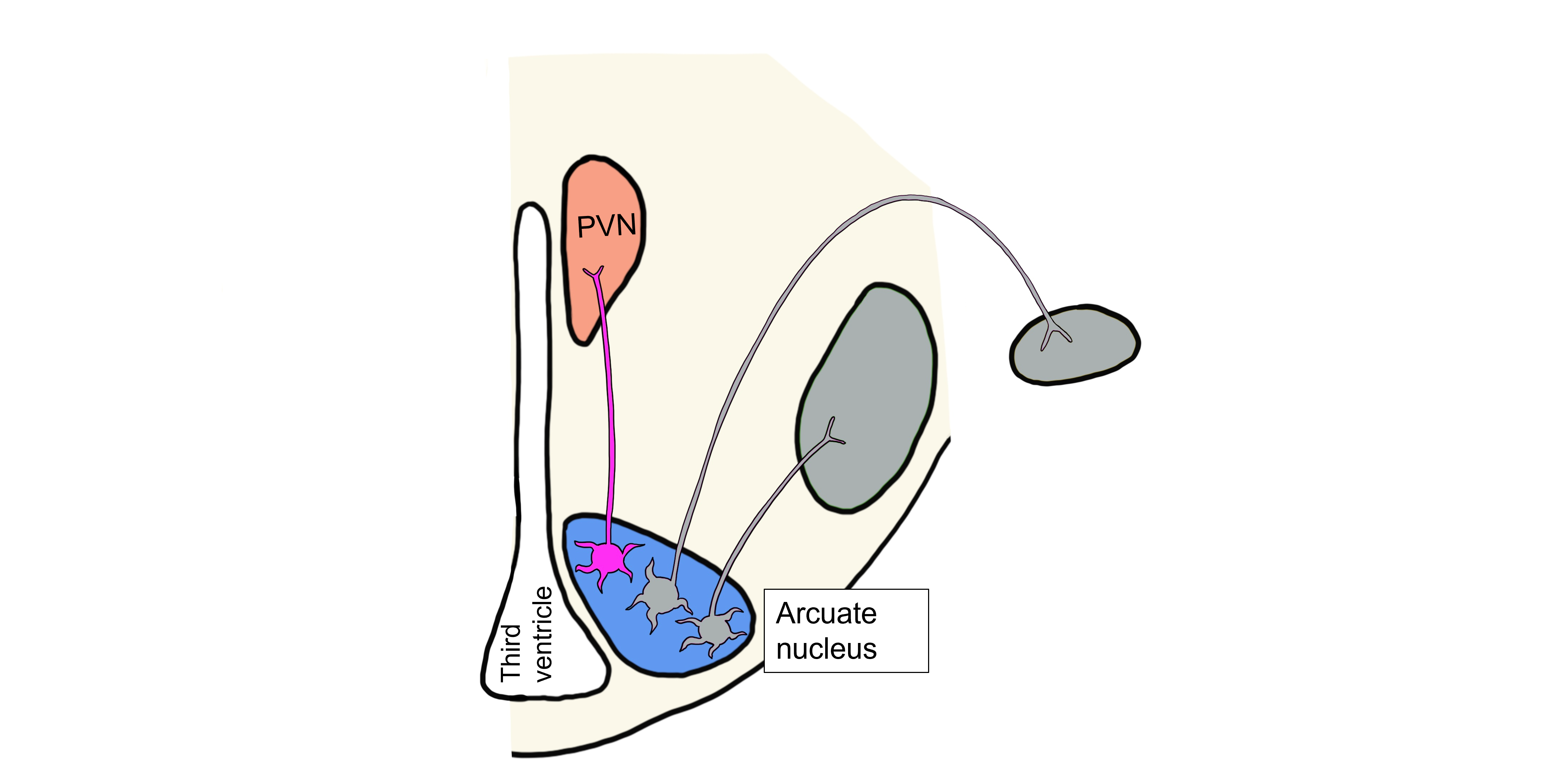
Visceromotor Response to Decreased Leptin
When leptin levels are low, arcuate neurons are activated that project to the brainstem and control the hypothalamic visceromotor response. These arcuate neurons release NPY and AgRP onto brainstem neurons that will act to increase parasympathetic nervous system activity, decreasing the metabolic rate and decreasing body temperature.
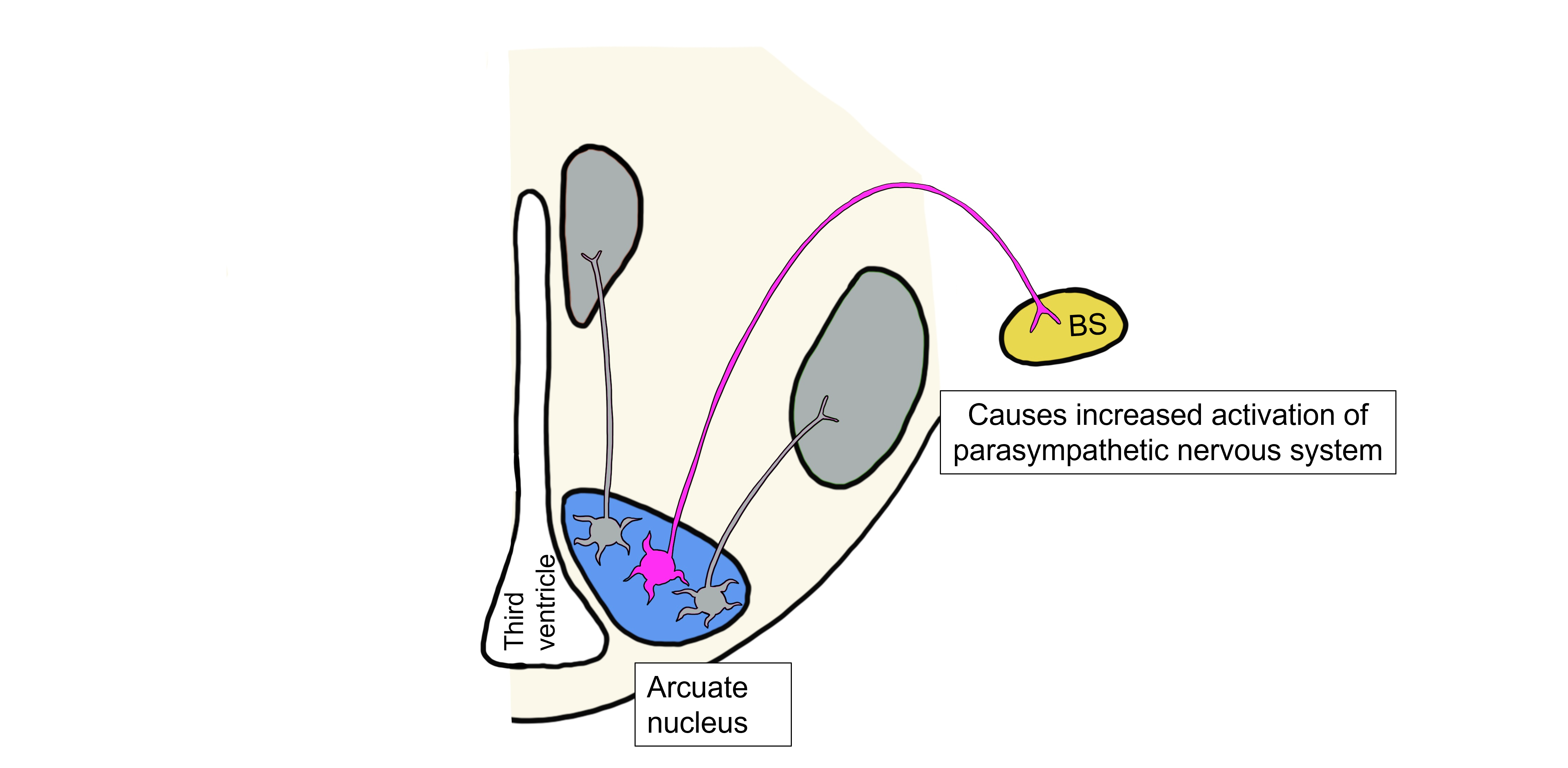
Somatic Motor Response to Decreased Leptin
Lastly, when leptin levels are low, the drop in leptin activates a population of arcuate neurons that project to the lateral hypothalamus and control the hypothalamic somatic motor response. These arcuate neurons release NPY and AgRP onto lateral hypothalamus neurons. NPY and AgRP stimulate the lateral hypothalamus, stimulating feeding behavior.
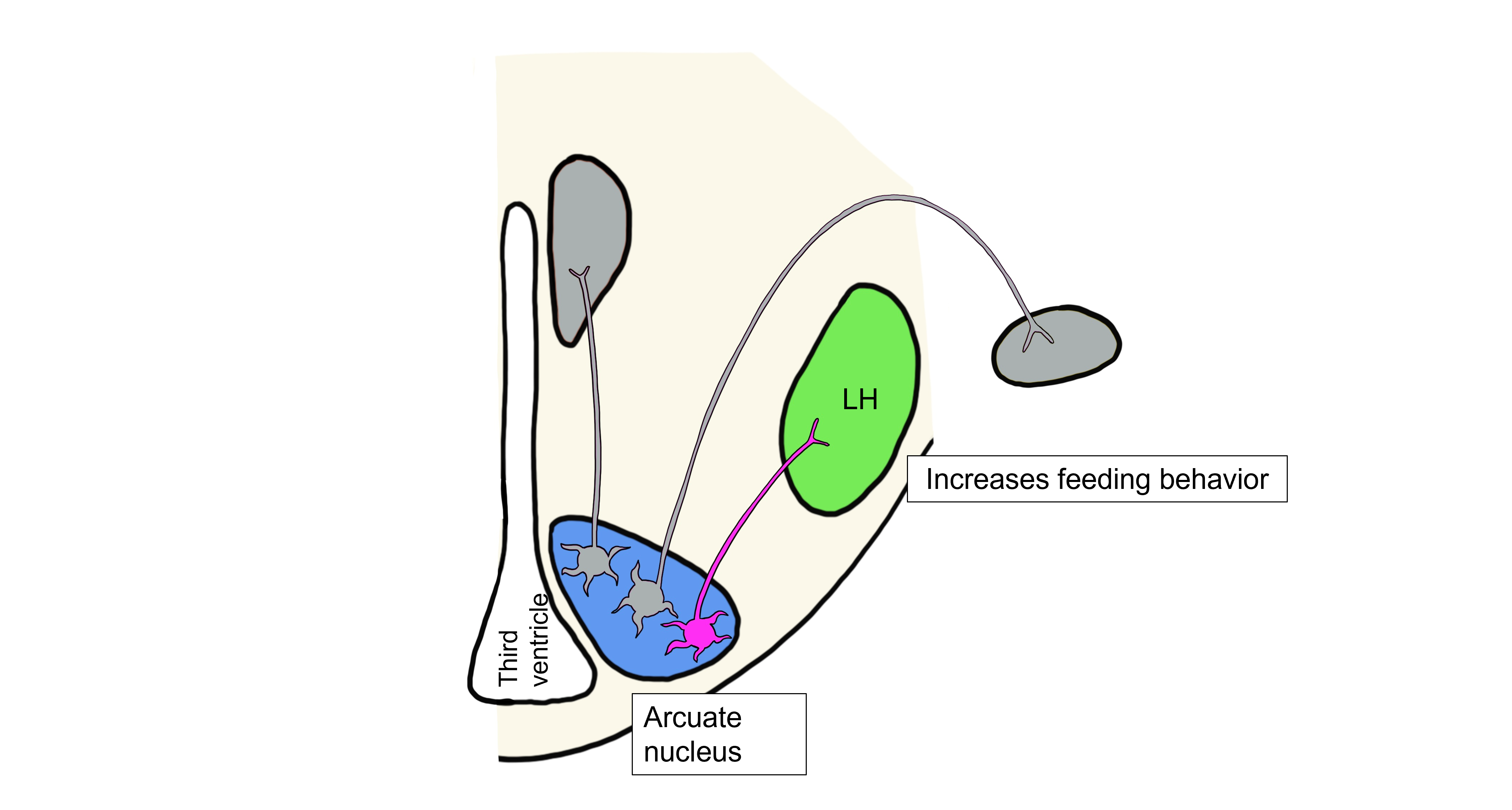
Overall, the decrease in metabolic rate, decrease in body temperature, and increased feeding brought about by the three hypothalamic responses will all ultimately lead to an increase in body fat and thus an increase in leptin levels, bringing fat and leptin levels back within the physiological setpoint range.
NPY and AgRP are examples of orexigenic signals. Orexigenic signals increase feeding behavior and increase body weight in response to changes in adiposity. AgRP and αMSH are considered antagonistic neurotransmitters because they both bind to the MC4 receptor on hypothalamic neurons, but exert opposite effects on feeding behavior.
Key Takeaways
- Motivated behaviors are voluntary behaviors that individuals find rewarding.
- Certain behaviors or stimuli are naturally rewarding because they are necessary for the survival of a species.
- The hypothalamus has three responses to help maintain homeostasis: humoral response, visceromotor response, and somatic motor response.
- Leptin levels are controlled by fat levels in the body.
- Leptin interacts with 2 different populations of neurons within the arcuate nucleus of the hypothalamus to bring about homeostasis.
Attributions
This chapter is adapted from “Motivated Behavior: Feeding Behavior” in Introduction to Neuroscience by Valerie Hedges which is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Media Attributions
- Hypothalamus Coronal © Casey Henley is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- PosteriorPituitary © Casey Henley is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Anterior Pituitary © Casey Henley adapted by Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Energy balance © Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- ob/ob mouse © BigPlankton is licensed under a Public Domain license
- Hypothalamic brain areas involved in feeding © Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- High Leptin aMSH and CART neurons © Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- High Leptin Humoral © Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- High Leptin Visceromotor © Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- High Leptin Somatic Motor © Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Low Leptin NPY and AgRP neurons © Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Low Leptin Humoral © Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Low Leptin Visceromotor © Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Low Leptin Somatic Motor © Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
maintenance of the body’s internal environment within a physiological range
pituitary stimulating hormones
'Fight or Flight' system
'Rest and Digest' system
hormone secreted by fat cells
signals that decrease feeding behavior
signals that increase feeding behavior